Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
CES Medicina Veterinaria y Zootecnia
versión On-line ISSN 1900-9607
Ces. Med. Vet. Zootec. vol.8 no.2 Medellín jul./dic. 2013
Evaluation of screening tests for antimicrobial residues in milk from individual cows treated with a combination of penicillins G and streptomycin*
Valoración de pruebas de campo para detección rápida de residuos antimicrobianos en leche individual de vacas tratadas con una combinación de penicilinas y estreptomicina
Valoração de testes de campo para detecção rápida de resíduos antimicrobianos em leite individual de vacas tratadas com uma mistura de penicilinas e estreptomicina
Violeta Díez1*, Zoot; Juan E. Pérez1, MSc; Martha Olivera1, MV, MSc, PhD; Juan G. Restrepo1, MV, PhD; David Villar1, MV, MSc, PhD.
* Corresponding autor: Violeta Diez BIOGENESIS Research Group, Faculty of Agricultural Sciences, Universidad de Antioquia, AA 1226, Medellín, Colombia. Email: violetadb@hotmail.com
1 BIOGÉNESIS Research Group, Faculty of Agricultural Sciences, Universidad de Antioquia, AA 1226, Medellín, Colombia.
(Recibido: 17 de octubre, 2013; aceptado: 22 de noviembre, 2013)
*Para citar este artículo: Díez V, Pérez JE, Olivera Ángel M, Restrepo JG, Villar D. Evaluation of screening tests for antimicrobial residues in milk from individual cows treated with a combination of penicillins G and streptomycin. Rev CES Med Zootec. 2013; Vol 8 (2): 52-60.
Abstract
Background: in Colombia, the law (Resolution 1382, 2013) prohibits the sale of milk that contains any antimicrobial drug residues, although no specific official screening tests and detection limits have been specified. At present, milk with positive results to both the Delvotest® and Snap® assays is simply rejected. To avoid contaminating bulk tanks with milk from individually treated cows, producers would benefit from having on-farm screening tests to conduct their own quality controls. In addition, on-site testing would allow farmers to check if the withdrawal times of commercially-available generic products are in accordance with labeled recommendations. Material and Methods: In this study, two commonly used rapid detection tests (Delvotest® and SNAP® specific for beta-lactams) were used on milk from 39 subclinical mastitic Holstein cows that were prescribed with daily intramuscular injections of a commercial suspension containing 8.000.000 IU of penicilin G (75% procaine penicilin G, 25% potasium penicillin) and 8 g of streptomycin sulfate, for a total of 4 days. Cows were individually milked and samples collected every 12 hours the day before, and for 3 days after the recommended withdrawal time of three days post-treatment. To inactivate the potential action of natural inhibitors of microbial growth that may be present in milk (ie., lysozyme and lactoferrin), the results of the Delvotest® were compared before and after milk samples were subjected to heat treatment (82°C for 5 minutes). Results: When the Delvotest® was used as per manufacturer's instructions (i.e., without heating), 7 of 39 cows were positive for one more day past the recommended withdrawal period. However, the results of the Snap® specific for beta-lactams and Delvotest® post-heating showed that only 2 of those 7 cows were positive, suggesting that 5 animals gave false positive results. For the 312 milk samples analyzed, a high degree of concordance was observed (Kappa coefficient=0.74±0.1) between the Snap® and Delvotest® post-heating. Conclusions: Considering that the streptomycin in this product is known to be eliminated faster than penicillin-G, the results suggest that the efficacy of the Delvotest® (after heat treatment) is similar to that of the Snap® beta-lactams for the detection of penicillin residues. However, when the Delvotest® was not preceded by heat treatment to inactivate potential natural inhibitors, it yielded a high number of false-positive results. The results also showed that in 95% (37/39) of the cows treated with this commercial product, the labeled instructions of a 3 day withdrawal period were adequate for compliance within the law.
Key words: delvotest, milk, Penicillin G, residues, SNAP, Streptomycin.
Resumen
En Colombia, la ley (Resolución 1382, 2013) prohíbe la venta de leche que contenga residuos de cualquier medicamento antimicrobiano, aunque no se especifican pruebas oficiales ni límites de detección que deban cumplir. Actualmente, la leche que emite resultados positivos a los kits del Delvotest® y Snap® simplemente es rechazada. Para evitar contaminar tanques de acopio con leche de vacas tratadas, los productores se beneficiarían de tener pruebas in situ que les permitan hacer sus propios controles. Además, ello permitiría comprobar si los tiempos de descarte de productos comerciales cumplen con las recomendaciones de los insertos. En este estudio se evaluaron dos pruebas de detección rápida (Delvotest® y SNAP® específico parar beta-lactámicos) en leche de 39 vacas con mastitis subclínica que fueron tratadas con inyecciones diarias intramusculares de una suspensión comercial de 8.000.000 UI de penicilina G (75% penicilina procaínica G, 25% penicilina potásica) y 8 g de sulfato de estreptomicina, durante 4 días. Las vacas se ordeñaron manualmente y recolectaron muestras de leche, cada 12 horas, por 1 día antes y 3 después del tiempo de retiro recomendable de 3 días post-tratamiento. Para inactivar la acción de inhibidores naturales del crecimiento bacteriano que pueden estar presentes en leche (ej, lisozima y lactoferrina), los resultados del Delvotest® se compararon antes y después de que las muestras de leche fuesen sometidas a calentamiento (82°C por 5 minutos). Cuando el Delvotest® se usó de acuerdo a las instrucciones de la compañía, es decir, sin calentamiento, 7 de 39 vacas dieron positivas por ≥ 1 días pasado el tiempo de retiro recomendado. Sin embargo, los resultados del Snap® y Delvotest® post-calentamiento mostraron que sólo 2 de las 7 vacas eran positivas, sugiriendo que 5 animales estaban dando falsos positivos. En las 312 muestras de leche analizadas se obtuvo un alto grado de concordancia (coeficiente Kappa = 0.74±0.1) entre el Snap® y el Delvotest® post-calentamiento. En conclusión, los resultados sugieren que la eficacia del Delvotest® (post-calentamiento) es similar a la del Snap® específico para beta-lactámicos en lo que respecta a detección de residuos de penicilina. Sin embargo, cuando el Delvotest® no iba precedido de calentamiento para inactivar inhibidores naturales, se produjeron demasiados falsos positivos. Los resultados también mostraron que en el 95% (37/39) de las vacas tratadas con este producto, la recomendación de descarte por 3 días cumplía con la legislación vigente de no contener residuos.
Palabras clave: delvotest, estreptomicina, leche, Penicilina G, residuos, SNAP.
Resumo
Na Colômbia, a lei (Resolução 1382 de 2013) proíbe a venda do leite que tenha resíduos de qualquer medicamento antimicrobiano, embora não se especifiquem testes oficiais nem limites de detecção que devam se cumprir. Atualmente, o leite que emite resultados positivos aos Kits de Delvotest® e Snap® simplesmente é rejeitado. Para evitar contaminar os tanques de armazenamento com leite de vacas tratadas, os produtores beneficiar-se-iam de ter os testes in situ que lhes permita fazer seus próprios controles. Além, isto permitiria comprovar se os tempos de retiro do leite ao utilizar produtos comerciais cumprem com as recomendações da vide bula. Neste estudo avaliaram-se dois testes de detecção rápida (Delvotest® e SNAP® especifico parar beta-lactâmicos) no leite de 39 vacas com mastite subclínica que foram tratadas com injeções diárias intramusculares de uma suspensão comercial de 8.000.000 UI de penicilina G (75% penicilina procaína G, 25% penicilina potássica) e 8g de sulfato de estreptomicina durante quatro dias. Extraiu-se o leite das vacas com ordenha manual e se fez uma amostragem de leite (cada 12 horas) um dia antes e três dias depois do tempo de retiro do leite, que tinha como recomendável, na vide bula, três dias após final do tratamento. Para inativar a ação de inibidores naturais do crescimento bacteriano, que possam estar presentes no leite (ex. lisozima e lactoferrina), os resultados do Delvotest® compararam-se antes e depois de que as amostras de leite fossem sometidas a aquecimento (82°C durante 5 minutos). Quando o Delvotest® usou-se de acordo com as instruções da companhia, quer dizer, sem aquecimento, sete das 39 vacas deram positivas por ≥ 1 dia passado o tempo de retiro recomendado. Embora, os resultados do Snap® e Delvotest® após aquecimento do leite mostraram que só dois das sete vacas eram positivas, sugerindo que cinco animais estavam apresentando falsos positivos. Nas 312 amostras de leite analisadas obteve-se um alto grau de concordância (coeficiente Kappa = 0.74±0.1) entre o Snap® e o Delvotest® após aquecer o leite. Em conclusão, os resultados sugerem que a eficácia do Delvotest® (após o aquecimento do leite) é similar á do Snap ou especifico para beta-lactâmicos no que respeita a detecção de resíduos de penicilina. Embora, quando o Delvotest® não ia precedido do aquecimento do leite para inativar inibidores naturais, produziram-se muitos falsos positivos. Os resultados também demonstraram que o 95% (37/39) das vacas tratadas com este produto, estavam de acordo com a recomendação de descarte por três dias e cumpria com a legislação vigente de não conter resíduos.
Palavras chave: delvotest, penicilina, resíduos em leite, snap test.
Introduction
The Colombian legislation does not allow bulk tank milk to be sold with detectable levels of antimicrobials (Resolution 1382, 2013) and current policies with regard to food safety issues for milk and meat products are intended to comply with strict regulations and to allow competition in a free market(1). However, most farm operations do not conduct on-site tests for the detection of antimicrobial residues in milk and/or urine, and simply rely on the instruction given by the pharmaceutical company of a commercial drug to decide on the time to withdraw milk or send the animal to slaughter. There are two particular situations in which both the veterinarian and producer would benefit from using on-farm screening tests: a) extra-label use of a veterinary drug (ie., different dosage, route of administration, intended animal species, etc.); and b) pathologies that could alter the clearance of drugs by increasing its half-life of elimination(2). On-site screening for antimicrobials has been long implemented in most NorthAmerican and British dairy operations(3, 4) and is recommended by the "Pasteurization Milk Ordinance" to certify grade A milk(5).
At present, there are dozens of commercially available rapid detection assays based on different analytical principles, but none is 100% reliable and although they may have been certified and officially approved for use in tank bulk milk, their performance in individual cow milk may be different. The majority of them detect levels of antimicrobials that are usually below permissible levels established by the Codex Alimentarius, which makes them highly sensitive(6). In countries like Colombia, where the law stipulates that milk should be devoid of any residues, using very sensitive assays guarantees that milk will not yield false-negative results. By contrast, these assays, and particularly those based on microbial growth inhibition, tend to be less specific and it has been proved that many will yield false positive results(7), which would unfairly result in milk being discarded and a financial loss to the producer. According to an expert group from the University of California, the ideal assay/s to choose should be based on "predictive values" attained from preliminary studies on the type of antimicrobials and prevalence of residues in the area(8).
The Delvotest® test is based on the inhibition of the rapid growth and acid production of the microorganism Bacillus stearothermophilus in an agar medium during a 3 hour incubation period at 64°C. As such, it detects numerous antimicrobials and at thresholds generally close or below minimum permissible residue levels established by the Codex Alimentarius. This makes it very sensitive but of very low specificity. By contrast, the Snap® uses an immuno-enzymatic method which targets antimicrobials of the beta-lactam family (penicillin G, amoxycillin, ampicillin, ceftiofur, cephapirin, etc). The objective of this study was to compare the results of these two commonly used rapid detection assays in individual milk from cows treated for mastitis with a commercially available combination of procaine penicillin-G and streptomycin.
Materials and methods
Experimental cows and treatment
The study was conducted in a dairy farm that has been officially accredited by the Colombian Institute of Agriculture with the equivalent to the grade A certification by the Pasteurized Milk Ordinance. The farm is comprised of about 400 Holstein cows and located on the high tropical plains of Antioquia, with pastures consisting of kikuyu grass (Penissetum clandestinum). Animals that need to be treated for mastitis or other pathologies (ie., respiratory, lameness) are placed in a separate pasture lot and manually milked twice a day. Throughout a 3 month period in 2011, a total of 39 animals at different stages of lactation and parity that received a similar treatment for subclinical mastitis were included in the study. Isolation of pathogens was not routinely conducted at the farm, and so, the etiology of the mastitis was unknown. Treatment to each animal consisted of daily intramuscular injections with a commercial suspension (Vicarpen®, Vicar laboratories) containing 8.000.000 UI of penicilin G (75% penicilin G-procaine, 25% potasium penicillin) and 8 g of streptomycin sulfate, for a total of 4 days. For a 500 kg cow, this was approximately equivalent to daily dosages of 12.000 UI/kg PPG, 4000 UI/kg potasium penicillin and 16 mg/kg streptomycin. The product was reconstituted immediately before use in 20 mL sterile saline, and injected deep intramuscularly in two equal volumes of 10 mL at each buttock. Typically, cows that recovered after the withdrawal period were returned to their original lots with the rest of the herd and joined the milking regular schedule in pallor rooms; however, those used in the experiment were kept for an additional 3 days beyond the recommended withdrawal time to facilitate the collection of milk samples.
Experimental design
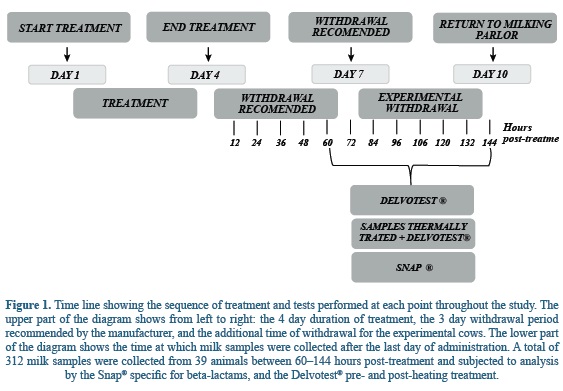
The experimental design is graphically shown in figure 1. On day 1, administrations were started for each cow and continued until day 4. On day 7, the withdrawal time for milk ended according to the product labeled instructions of 3 days following the last administration. On day 10, and for the purpose of the study, cows that were manually milked for an additional 3 days past the usual time of withdrawal were returned to their original destination lot. Milk samples were collected every 12 hours between 60 and 144 hours after the last administration and submitted for residue analysis by the Snap® and Delvotest® pre- and post-heating treatment.
Milk collection and residue analysis
All four quarters were manually milked by farm operators in stainless steel buckets from which a composite sample was directly collected into 10 mL sterile plastic vials. To avoid potential contamination between samples, buckets and hands were washed with an iodine solution and thoroughly rinsed between cows. The samples were kept at 4°C for a maximum of 2 days prior to analysis.
The Delvotest® SP (DSM Food Specialties, Netherlands) and Snap® Beta-lactam (Aqualab SA, Colombia) tests were conducted according to the recommendations of the manufacturers, except that the Delvotest was retested after milk was subjected to heat treatment of 82°C for 5 minutes. This treatment has been shown to be a fast, simple, and inexpensive way to remove false-positive results due to natural inhibitors and has no effect on positive samples containing most antimicrobials(9). According to the manufacturers, both methods can detect concentrations of 2-4 µg/kg of penicillin in milk, which are below the maximum residue limit of 4 µg/Kg established by the Codex Alimentarius.
Statistics
To assess the extent of agreement between assays, the results (presence or absence of residues) were represented on 2 X 2 contingency tables and the Kappa Coefficient (k) was calculated as described by Landis and Koch(10) using the formula:
K = [(Σ concordances observed)-(Σ random concordances)] [(Total number of observations)-(Σ random concordances)]
The interpretation of k was performed by correlating its value with a qualitative scale, which included six levels of strength: poor (<0.01), slight (0.01-0.20), fair (0.21-0.40), moderate (0.41-0.60), high (0.61-0.8), perfect (0.81-1.0).
Results
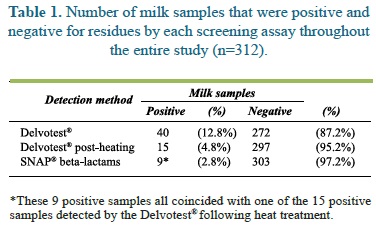
Table 1 shows the numbers of positive and negative milk samples for each one of the screen assays in all 312 milk samples that were taken throughout the study. The Delvotest® detected 40 (12.8%) positive samples, 25 of which become negative when retested following heat treatment. Of the 15 (4.8%) remaining positive samples, the SNAP yielded positive results in 9 (2.8%), this test was otherwise negative in all samples where the Delvotest was also negative.
The percentage of cows that were positive at each time point tested is shown in figure 2. When the Delvotest® was not preceded by heat treatment the percentage of cows still yielding positive results at 12, 24, 36 48 and 60 hours past the recommended withdrawal time (at 72 hr post-administration) was 18% (7/39), 15% (6/39), 13 (5/39), 10 (4/39) and 3% (1/39) respectively. However, when the Delvotest® was preceded by heat treatment only 8% (3/39) were positive for one more milking (at 84 hr post-administration), and only 2 of them were also positive to the Snap® beta-lactams.
To compare the results of the Delvotest® post-heating and the Snap® assays, the presence or absence of residues for each test were tabulated in a 2x2 contingency table as shown in Table 2. The proportion of concordances observed among the total number of observations, after excluding all random concordance, was high (kappa ±S.D = 0.74 ± 0.10), showing that both assays yielded similar results.
Discussion
The objective of this study was to assess the performance of common screening tests used for the detection of antimicrobial drug residues in individual milk from cows treated for subclinical mastitis with one of several dozen commercially available procaine penicillin-G products (PPG) in the Colombian market. Both the Delvotest® and Snap® specific for beta-lactams were selected based on being the two most routinely employed assays by the Colombian dairy industry. The results showed that the labeled withdrawal time of 3 days after the last administration was adequate in 37 of 39 cows, and only 2 animals yielded milk positive for residues to both tests for an additional day. However, the Delvotest® when used as instructed, that is, without heat treatment, resulted in a high number of false positive results. Of a total of 40 positive samples to the Delvotest®, only 15 remained positive when retested after heating at 82º C for 5 minutes, and of those, only 9 tested positive by the Snap® specific for beta-lactams. It has long been known that cows with mastitis yield milk with natural microbial growth inhibitors, such as lysozyme and lactoferrin(11, 12). In particular, tests such as the Delvotest®, which is a detection assay based on microbial growth, have been shown to yield false positive results due to the presence of such natural inhibitors(13).
The implications of considering the Delvotest®, without previous heat treatment, as a definitive test by the Colombian dairy industry is likely to be causing milk tanks being erroneously discarded. To avoid such risk, more specific tests should be used in combination with the Delvotest®; in this respect, the results of the present study showed that the Snap specific for beta-lactams could meet the needs for being that other assay. In fact, when the Delvotest® was retested on heated samples, the concordance with the results of the Snap® were very high (kappa coefficient=0.74), implying that either technique should provide very similar outcomes. Unfortunately, since neither the Delvotest® nor the Snap® can be considered as gold standard assays for the detection of antimicrobials, the sensitivity, specificity and predictive values could not be calculated for either test, obviously that would be essential to determine how accurate and predictive they truly are.
In general, drugs with milk withdrawal periods of more than 4 days are not approved for dairy cows in lactation. In Colombia, there are numerous procaine penicillin-G products that are registered for use in lactating cows, and the times of milk withdrawal for the majority are 2-3 days, and exceptionally 4 days(14). For the dosage and duration of treatment used in this study, the withdrawal time was not expected to exceed 3 days. In fact, the FDA (Food and Drug Administration) recommends withdrawal times for IM PPG products (not exceeding 10 mL per injection site) of 2, 3, 4 y 5 days for dosages of 6,600, 14000, 21.000 y 28,000 UI/kg respectively(15). Therefore, the suggested recommendation of a 3 day withdrawal time for the dosage of ≈12,000 UI/kg used in this study was adequate, and in fact, only 2 out 39 cows yielded positive results for one more day. In other studies that evaluated the presence of milk residues beyond the recommended time of withdrawal to multiple types of antimicrobial drugs, penicillin was observed to account for the majority of positive cases(16). Potential reasons that can explain a prolonged presence of drug residues in milk include different forms of extralabelled administrations. For procaine-penicillin-G, all commercial products are aqueous suspensions recommended for intramuscular administration but contraindicated by the subcutaneous route. It has been shown that subcutaneous injections of procaine-penicillin-G will cause local hematomas, inflammation, and fibrosis, all of which can result in delayed absorption from the injection site and consequent persistent excretion in milk(17,18). Delayed absorption was also shown to occur when large volumes of more than 20 mL were injected in the same location. In this study, the maximum volume injected per site did not exceed 10 mL, as recommended by FDA(15).
With respect to the presence of streptomycin sulfate in the commercial formulation, it is known that at the dosage given, this other antimicrobial drug is more rapidly eliminated than PPG(19-21).Therefore, any positive results on the Delvotest® beyond the day of withdrawal would be most likely caused by PPG and not streptomycin. Studies that evaluated milk residues of streptomycin following daily IM administrations of 10 mg/kg for 3 days, found that at 24 hours post-injection, the concentrations where below the minimum permissible level of 200 µg/kg established by the Codex Alimentarius, and which is already below the detection limit of the Delvotest®(22). Commercial products that are only based on streptomycin sulfate at the dosage given in this study have a milk withdrawal period of only 2 days, for that reason no specific assay was considered necessary to detect this antimicrobial.
Conclusion
This study showed that two residue screening tests, the Delvotest® after heat treatment and the Snap® specific for beta-lactams can be useful and fast screening tests to verify that cows treated with combinations of PPG-streptomycin are no further excreting penicillin. Because some cows continued excreting penicillin past the point of withdrawal time, conducting these tests by producers, as a common tool of good husbandry practices, could avoid contaminating entire bulk tanks. Either the Delvotest® or the Snap® produced similar results and can be recommended interchangeably when a penicillin based product has been applied. In Colombia, the Delvotest® is the main and sometimes only test used by dairy companies; this is likely causing numerous false positive outcomes that should be cross referenced with a more specific assay.
Referencias
1. Conpes (COLOMBIA). Consolidación de la Política sanitaria y de inocuidad para las cadenas láctea y Cárnica. Consejo nacional de política y Social. Departamento Nacional de Planeación. Bogotá; 2010. [ Links ]
2. Riviere JE, Webb AI, Craigmill AL. Primer on estimating withdrawal times after extralabel drug use. FARAD Digest. J Am Vet Med Assoc 1998; 213:966-968. [ Links ]
3. Gibbons-Burgener SN, Kaneene JB, Lloyd JW, Leykam JF, Erskine RJ. Reliability of three bulk-tank antimicrobial residue detection assays used to test individual milk simples from cows with mild clinical mastitis. J Am Vet Med Assoc 2001; 62: 1716-1720. [ Links ]
4. Hillerton JE, Halley BI, Neaves P, Rose MD. Detection of antimicrobial substances in individual cow and quarter milk samples using Delvotest microbial inhibitor tests. J Dairy Sci 1999; 82: 704-711. [ Links ]
5. PMO (USA). Drug Residue Testing and Farm Surveillance. Grade "A" Pasteurized Milk Ordinance; 2007. [ Links ]
6. Toldra F, Reig M. Methods for rapid detection of chemical and veterinary drug residues in animal foods. Trends in Food Science & Technology 2006; 17: 482-489. [ Links ]
7. Andrew SM, Frobish RA, Paape MJ, Maturin LJ. Evaluation of selected antiobiotic residue screening tests for milk from individual cows and examination of factors that affect the probability of false-positive outcome. J Dairy Sci 1997; 80: 3050-3057. [ Links ]
8. Gardener IA, Cullor JS, Gales FD. Alternatives for validation of diagnostic assays used to detect antibiotic residues in milk. J Am Vet Med Assoc 1996; 209: 46-52. [ Links ]
9. Kang JH, Jin JH, Kondo F. False-positive outcome and drug residue in milk samples over withdrawal times. J Dairy Sci 2005; 88:908-913. [ Links ]
10. Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159-74. [ Links ]
11. Carlsson A, Bjorck L, Persson K. Lactoferrin and lysozyme in milk during acute mastitis and their inhibitory effect in Delvotest P. J Dairy Sci 1989; 72:3166-3175. [ Links ]
12. Van Eenennaam L, Cullor JS, Perani L, Gardner IA, Smith WL, Dellinger J, Guterbocks WM. Evaluation of Milk Antibiotic Residue Screening Tests In Cattle with Naturally Occurring Clinical Mastitis. J Dairy Sci 1993; 76:3041-3053. [ Links ]
13. Jevinova P, Dudrikova E, Sokol J, Nagy J, Mate D, Pipova M, Cabadaj R. Determination of oxytetracycline residues in milk with the use of HPLC method and two microbial inhibition assays. Bull Vet Inst Pulawy 2003; 47:211-216. [ Links ]
14. Thomson PLM. Diccionario de especialidades farmacéuticas. 54 Ed. Ediciones PLM: México; 2008. [ Links ]
15. Payne MA, Craigmill A, Riviere JE, Webb AI. Extralabel use of penicillin in food animals. J Am Vet Med Assoc 2006; 229:1401-1403. [ Links ]
16. Seymour EH, Jones GM, Mcgilliard ML. Persistence of Residues in Milk Following Antibiotic Treatment of Dairy Cattle. J Dairy Sci 1988; 71:2292-2296. [ Links ]
17. Korsrud GO, Boison JO, Papich MG, Yates WDG, MacNeil JD, Janzen ED, Cohen RDH, Landry DA, Lambert G, Yong MS, Messier JR. Depletion of intramuscularly and subcutaneously injected procaine penicillin G from tissues and plasma of yearling beef steers. Can J Vet Res 1993; 57: 223-230. [ Links ]
18. Dubreuil P, Daigneault J, Couture Y, Gua P, Landry D. Penicillin concentrations in serum, milk, and urine following intramuscular and subcutaneous administration of increasing doses of procaine penicillin in lactating dairy cows. Can J Vet Res 2001; 65:173-180. [ Links ]
19. KuKanich B, Gehring R, Craigmill AL, Riviere JE. Effect of formulation and route of administration on tissue residues and withdrawal times. J Am Vet Med Assoc 2005; 227:10. [ Links ]
20. Mercer HD, Geleta JN, Carter GG. Absorption and excretion of penicillin G from the mastitic bovine udder. J Am Vet Med Assoc 1974; 164: 613-617. [ Links ]
21. Mercer HD, Geleta JN, Porteous LA, Condon RJ. Excretion of penicillin G and dihydrostreptomycin from quarters of cows with experimental induced staphylococcal mastitis. Am J Vet Res 1974; 35: 1191-1196. [ Links ]
22. JECFA. Summary of evaluations performed by the Joint FAO/WHO Expert Committee on Food Additives: ONU; 2002. [ Links ]