Introduction
In many cases, climate defines the geographical distribution of a species, the types of vegetation, and the appropriate management of the crops, demonstrating climate's great influence on the growth of plants (Dey et al., 2016). Negative influences on food security include climate variability (CV), climate change (CC) manifested through global warming, the change in rainfall patterns, and the increased incidence of extreme weather events (IPCC, 2019). In addition, the effects on crops are more intense in low latitude countries, with higher initial temperatures (Fischer et al., 2022; Fischer, Orduz-Rodríguez et al., 2022), and even more so in marginal or already degraded soils and in underdeveloped regions with insufficient potential for adaptation to these changes (Yohannes, 2016). Extreme events due to flooding of agricultural land will continue to increase, including in regions where previously it was not expected (Fischer, 2021). Contradictorily, in northern areas CC can have effects, mostly positive, manifested in an increase in productivity and diversity of cultivated varieties, unlike southern areas where the inconveniences of CC prevail with lower usable yields, greater instability in plant yield of cultivated plants, and decreases in areas suitable for traditional crops (Potopová et al., 2015).
In these scenarios, not only at a local level but also globally, the intensity and frequency of abiotic stress in plants constantly increases (Dubey et al., 2021). Recent studies reveal that the multifactorial stresses generated by CC and global warming cause severe decreases in plant growth and survival and biodiversity of the microbiome (Zandalinas et al., 2021).
Sthapit et al. (2012) affirm that global warming will have a significant impact on small farmers who depend on rainfed agriculture. Likewise, increases in the frequency of coastal flooding induced by CC can significantly reduce the area available for agriculture (Sthapit et al., 2012). In general, adaptations of fruit species to CC require time and long-term investments (Chmielewski et al., 2008).
A shortage of precipitation because of recent CC is expected. However, most fruit growing regions experience almost constant precipitation (Drogoudi et al., 2020) with a slight shift of more rain during certain periods than in other periods of the year (Bisbis et al., 2018). A similar situation occurs in the Colombian Andes with an increase in rainfall in the highlands (Hirabayashi et al., 2013). Long-term (60 year) weather records show a change from moderate rainfall to an increase in the incidence of heavy rainfall (> 20 mm) (Kunz & Blanke, 2022) that sometimes leads to flooding.
Fruit production can increase in some areas due to increased rainfall, but in other areas, production can fall seriously due to decreased rainfall (Sthapit et al., 2012). In general, it is estimated that fruit trees will be less affected by CC compared to semi-annual or annual crops (e.g., cereals) (Sthapit et al., 2012); however, fruit species in temperate zones will move to new areas closer to the poles (Jones et al., 2005), while tropical fruit trees may extend over their latitudinal strip and/or to higher altitudes (Fischer & Melgarejo, 2020).
In recent years, because of CC and especially global warming (Hirabayashi et al., 2013), waterlogging and flooding have increased in frequency and are unpredictable all over the world (Zhang et al., 2021), especially due to erratic and non-seasonal rainfall (Dubey et al., 2021). Hirabayashi et al. (2013) model flood frequencies through global warming predictions and report a 42% increase in frequencies, compared to an 18% reduction in flooding globally. Flooding will increase across large areas of South and Southeast Asia, Northeast Eurasia, East and low-latitude Africa, and into South America, where flooding will increase especially in the northern part of the Andes, while decreasing in southern South America (Hirabayashi et al., 2013).
In Latin America, Wood et al. (2000) estimate that 13.3% of arable land suffers from poor drainage due to the physiography that favors flooding as well as high groundwater levels or stagnant surface water. The rainy seasons in soils with poor drainage produce anaerobic conditions that are harmful to the roots (Moreno & Fischer, 2014). In addition, Kaur et al. (2020) report other causes of waterlogging or flooding that include rainfall after irrigation, excessive irrigation, poor surface and internal drainage in heavy clay soil types as well as in coarse-textured topsoil over compacted clay subsoil. Plantations in the vicinity of large and small rivers are also at risk, as these often overflow; Larcher (2003) classifies these as phenomena that do not depend solely on CC.
Waterlogging reduces the quality of soils and the productivity of many crops (Singh et al., 2010), affecting large areas of the world (Martínez-Alcántara et al., 2012). And Kaur et al. (2020) characterize soil flooding as the most harmful abiotic stress that affects crops apart from drought.
Waterlogging in the rhizosphere of plants is influenced by several factors, but especially climate that affects the amount of water incorporated into the soil, the volume of water that passes through or over the soil surface, and the amount of soil water used by plants and other organisms (Kaur et al., 2020).
For the survival of many plants and microorganisms in the soil, oxygen (O2) is a crucial element; losses caused by this type of abiotic stress can affect up to 40% of the total production (Moreno & Fischer, 2014). The availability of O2 is much lower in stagnant water and generates much stronger stress conditions compared to moving water, since turbulence facilitates the solubilization of O2 in flooded water (Kreuzwieser & Rennenberg, 2014).
The availability of O2 for flooded roots decreases because water contains fewer gases than the atmosphere since the diffusion of these gases dissolved in water is 10,000 times slower than in air (Parent et al., 2008). This generates an energy crisis in the tissues subjected to hypoxia that can cause the death of a plant (Moreno & Fischer, 2014).
The objective of this review was to characterize the effect of waterlogging and flooding on fruit crops in the context of global warming and the probability of very abundant rains that can greatly harm the growth and development of crops. We emphasize tropical and subtropical species in order to facilitate decision-making for future research and to enhance fruit production better adapted to these new conditions.
Influence on the soil
Kreuzwieser and Rennenberg (2014) and Kaur et al. (2020) define flooding as water above the soil level, while waterlogging refers to water that has completely saturated the soil, leading to O2 deprivation since excess inflowing water displaces soil air from the soil pores (Revelo, 2020). This means that O2 concentration at the root surface decreases drastically since the O2 level of the water is markedly lower than that of the air, and the air in the atmosphere contains about 20% O2 (200 kg m-3) compared to <0.01 kg m-3 dissolved O2 in a flooded soil (Taiz et al., 2017).
Schaffer (2006) differentiates between normoxia (soil with sufficient O2 content), hypoxia (low O2 content in the soil), and anoxia (trace O2 content in the soil, like the case of prolonged waterlogging). The remaining O2 in the soil is consumed by microbial activity and plant roots (Kreuz-wieser & Rennenberg, 2014). Thus, in waterlogged soils, anoxia is always preceded by hypoxia (Drew, 1997).
In flooded soils, the redox potential decreases (Sanclemente et al., 2014), indicating that the low level of O2 affects the availability of nutrients for the plant (Unger, Kennedy et al., 2009; Unger, Motavalli et al., 2009). Flooding hinders the activity of enzymes such as ß-D-glucosidase and phosphatase, necessary in the carbon, nitrogen (N), sulfur (S) and phosphorus (P) cycles (Wang & Lu, 2006); and ethylene concentration increases (Malik et al., 2003). These conditions in the soil negatively affect the growth and development of plants (Jiménez et al., 2012).
During waterlogging, O2 deficiency changes the physico-chemical properties of soils considerably (Kreuzwieser & Rennenberg, 2014). On the one hand, the lack of O2 harms the microbial communities in the soil (Unger, Kennedy et al., 2009); and it reduces numerous oxidized nutrients as follow: (nitrate [NO3-], iron(III) [Fe3+], sulfate [SO4 2-]) and generates high levels of reduced compounds (iron (II) [Fe2+], manganese (II) [Mn2+], ammonium [NH4+], hydrogen sulfide [H2S]) and organic compounds (acids, carbonyls, alkanes, etc.). In many cases, these low oxygen changes are toxic to plants (Jackson & Colmer, 2005; Kreuzwieser & Rennenberg, 2014). The physicochemical properties change in their speed and magnitude according to the type of soil, duration of waterlogging or flooding and the prevailing environmental conditions, mainly temperature (Drew, 1997; Kozlowski & Pallardy, 1997).
The porosity of the soil is very important for the impact of waterlogging and is a determining factor in the availability of O2 for the roots (Moreno & Fischer, 2014). Waterlogged pores immediately generate O2 deficiency, and the remaining O2 is consumed very quickly by roots and microorganisms (Pallardy, 2008). Clay soils with their fine pores are particularly susceptible to O2 deficiency, compared to sandy soils that remain aerated, even within flowing water (Larcher, 2003). Waterlogging leads to O2 depletion in the soil, since the O2 diffusion rate in waterlogged soils is 10,000 times lower than in a well-drained one (Hossain & Uddin, 2011).
Flooded soils greatly limit the operation by machinery that can affect soil preparation, plant management, and crop harvesting, leading to lower yields (Kaur et al., 2020).
Influence on the plants
Plants are aerobic organisms that require O2 (Pucciariello & Perata, 2012) for the absorption of nutrients. Excessive moisture around the roots can cause lethal conditions (Lacona et al., 2012) in most terrestrial plants (Jiménez et al., 2012). Schaffer et al. (2009) report that the soil O2 level can be reduced from 20 to <5% within 1 to 2 d of waterlogging. To respond to O2 deprivation, plants have developed different morphological, anatomical, and metabolic adaptations to prevent cell damage in such hostile environments (Bailey-Serres & Voesenek, 2008; Xie et al., 2021). These are listed below.
Growth
Kozlowski and Pallardy (1997) mention that the most deleterious effects of waterlogging on woody plants are reduced root and stem growth, changes in nutrient uptake, and carbohydrate translocation that increases senescence and plant mortality. Crane et al. (2020) point out that the symptoms of waterlogging are very different in trees, reducing growth and yield, not only in non-tolerant but also in tolerant fruit species. Symptoms of waterlogging or excessively wet soils on fruit trees, gradually progress as follow: (1) wilting and scorching leaves; (2) fruit drop, leaf chlorosis and drop; (3) regressive death of the stem and limb dieback; (4) death of the tree (Crane et al., 2020).
The root zone is the region of the plant that is first and directly affected by an excess of water and O2 deficiency of the soils (Moreno & Fischer, 2014); root growth stops or the apices are injured or die (Larcher, 2003). This starts with the fine and fibrous roots (Fischer & Orduz-Rodríguez, 2012), because thicker roots are less susceptible to radial loss of O2 (Pedersen et al., 2020). Due to the anaerobic conditions in the rhizosphere, phytotoxic conditions increase from the accumulation of reduced ions and the products of anaerobic organisms that limit soil aerobic microorganisms and increase the presence of fungal pathogens such as Pythium, Phytophthora, and Fusarium (Fischer & Orduz-Rodríguez, 2012). In general, for fruit trees a water table of 1.5 m is the most recommended (Fischer & Orduz-Rodríguez, 2012).
Waterlogging affects stem growth according to the degree of adaptation of the plants. It can be reduced or accelerated (Blom & Voesenek, 1996) to position their flowers and leaves above the water level (Schopfer & Brennicke, 2010). However, in plants susceptible to waterlogging such as papaya (Carica papaya) (Crane, 2020), waterlogging for only 48 h seriously affects the growth in diameter and length of the stem and of the root (Khondaker & Ozawa, 2007).
Leaves react to waterlogging like plants exposed to water stress; stomatal conductance is reduced as well as the rate of elongation of the leaves (Lambers & Oliveira, 2019). As the time of exposure to hypoxic stress of the roots increases, the leaves yellow and necrosis increases, followed by their abscission. These symptoms are caused by the lack of uptake and transport of water and nutrients by the roots (Kozlowski & Pallardy, 1997; Aldana et al., 2014; Fischer et al., 2016). Interestingly, when apple (Malus domestica) trees are in full fructification, leaf-fall is less than that compared to trees without fruits (Lenz, 2009).
However, the growth and reproductive development of fruit trees that are not tolerant of waterlogging because of the inhibition of root respiration and the accumulation of toxic substances (Pan et al., 2021) is highly impaired. Processes as energetic as the formation of flower buds, flowering, and set and growth of fruits is inhibited, and the premature fall of these organs due to the suppression of photosynthesis and hormonal imbalance induced under anoxic conditions (Kozlowski & Pallardy, 1997) occurs. For example, peach (Prunus persica) fruits were smaller after waterlogging for 12 h per day for 8 weeks that produces ethylene and softening of the pulp earlier in postharvest than do fruits from non-waterlogged trees (Insausti & Gorjón, 2013).
Exposure to short-term hypoxia in the roots reduces the redox potential of the soil and can promote the availability of micronutrients in basic-reactive soils, by reducing Fe3+ to Fe2+ that can be metabolized by plants. This stimulates flowering and fruit production in carambola (Averrhoa carambola) (Schaffer et al., 2006).
Physiological considerations
Basic processes of development, physiology, and metabolism, such as cell division, respiration, growth, nutrient and water intake, and transpiration are O2-dependent processes; so O2 deprivation results in reduced growth (Sathi et al., 2022).
The anaerobic stress of the root suppresses its respiration, leading to energy wastage (Lambers & Oliveira, 2019), an increase in fermentation, acidification of the cytosol (cytoplasmic acidosis) and toxic effects due to the accumulation of ethanol, lactic acid, and acetaldehyde (Bailey-Serres & Voesenek, 2008; Xie et al., 2021) that can lead to root cell death (Drew, 1997). This energetic deterioration affects many metabolic processes, such as protein synthesis (Taiz et al., 2017). The amount of adenosine triphosphate (ATP) generated during fermentation is two ATP molecules per glucose molecule, produced by substrate phosphorylation (Taiz & Zeiger, 2010). Thirty-two ATP molecules are produced during oxidative phosphorylation (Jiménez et al., 2012) corresponding to a 16-times lower energy production through fermentation. Especially if photosynthesis is also decreased, an energy shortage is assumed under flooding conditions (Menezes-Silva et al., 2019). In general, plants suffering because of anaerobic conditions can switch their metabolism from aerobic respiration to the fermentation pathway, as an adaptive mechanism to oxygen deprivation through waterlogging conditions (Peña-Fronteras et al., 2008; Jiménez et al., 2012).
The result of this stress can be cell death in a few hours or days depending on the adaptation of the species to low O2 levels. In addition, if the stressed plant returns to normal O2 levels the recovery process can be endangered (Mielke & Schaffer, 2010; Cardona et al., 2016; Taiz et al., 2017). Roots under anaerobic stress do not suffer from the formation of reactive oxygen species (ROS) due to the absence of O2; instead, if the O2 concentration in the soil increases rapidly, a part is used to form ROS that generates oxidative damage to the root cells (Taiz et al., 2017).
The effect of waterlogging on decreases of the photosyn-thetic rate is great, especially in plants that are intolerant to this stress (Parent et al., 2008); it is primarily related to stomatal as well to nonstomatal limitations (Kreuzwieser & Rennenberg, 2014). Early decrease is associated with stomatal closure and consequently with the reduction of the photosynthetic process, also because of the reduction of leaf area (Pallardy, 2008). Pallardy (2008) characterizes the decrease in leaf area due to suppression in the formation and extension of leaves, as well as by leaf lesions and abscission. The reduction of the chlorophyll content and premature senescence of leaves contributes to an inhibition of photosynthesis in a later stage of waterlogging (Parent et al., 2008; Aldana et al., 2014).
Stomatal closure due to hypoxic stress of the root system has been observed in many species, e.g., lemon (Moreno & Fischer, 2014) and strawberry (Blanke & Cooke, 2004), known to remedy water loss (Taiz & Zeiger, 2010). In addition, flood-sensitive species appear to lack a mechanism to reopen stomata that have been closed by hypoxic soil conditions (Pallardy, 2008). Interestingly, waterlogging could not only increase stomatal resistance but also restrict water intake through reductions in root hydraulic conductivity (Else et al., 1995; Menezes-Silva et al., 2019), leading to an internal water deficit in the plant (Parent et al., 2008; Sanclemente et al., 2014).
Water transport within a plant is driven by a combination of three mechanisms: a) root pressure, b) cohesion, and c) suction forces generated by evaporation. The latter relies on the presence and functioning of open stomata and water vapor pressure deficit (VPD) between the plant and the ambient atmosphere (Taiz et al., 2017). Water channels are described as key regulatory proteins in cellular apoplastic water transport. They are also responsible for the response to changes in xylem water potential and are required for the transport of water (Lambers & Oliveira, 2019).
Studies of flooded strawberry plants have shown that leaf water potentials remain unchanged after flooding, without changes in water channel activity (Blanke & Cooke, 2004). In this case, turgor may be preserved by maintaining root pressure, an electrochemical and ion gradient, and xylem differentiation, assuming water channels remain open. This contrasts with drought-stressed strawberry plants, where water channel activity is reduced. In any case, the effect of flooding on water relations of strawberry stolons and leaves is less pronounced than that of drought (Blanke & Cooke, 2004). The stomatal closure under drought could be attributed to increased delivery of abscisic acid (ABA) from roots to the leaves (Lambers & Oliveira, 2019). However, Blanke and Cooke (2004) observe stomatal closure in strawberry leaves to be more rapid under flooding conditions than under drought; and they suggest this reaction is due to the release of stress ethylene because the ABA transfer from the roots to the leaves is severely depressed in the xylem.
By reducing stomatal conductance, plants can prevent water loss; however, this reaction also affects net photosynthesis under waterlogging stress (Davies & Flore, 1986). Taiz and Zeiger (2010) and Kozlowski and Pallardy (1997) characterize the decrease in photosynthetic activity due to waterlogged conditions by the following: 1) low water potential, 2) reduced stomatal conductance, 3) decreased activity of photosynthetic enzymes, 4) lower content of chlorophylls, and 5) irregular transport of photoassimilates that can alone or in combination with these mentioned reasons decrease photosynthesis. Additionally, Pan et al. (2018) and the other cited authors by them explain that during the waterlogging of the plants the leaf stomata close; and the degradation of chlorophyll causes yellowing and senescence of the leaves so that the capacity of the leaves to take advantage of light is reduced, generating a decrease in the photosynthetic rate.
The distributional pattern of photoassimilates can change under these conditions in the Chandler and Camarosa strawberry (Fragaria x ananassa) varieties, with an increase of 15.9% and 18.4% of the dry mass of the roots in waterlogged plants (Casierra-Posada & Vargas, 2007).
Anaerobic root conditions also affect hormone metabolism; root ethylene concentrations increase because this gas moves slower in flooded soil than in well-aerated soil, and because of greater production of this hormone under stress conditions (Lambers & Oliveira, 2019). Ethylene is a recognized signaling molecule that is involved in the acclimation of plants to hypoxia (Xie et al., 2021). Similarly, the synthesis of abscisic acid in flooded roots is a responsible signal in the leaves for their stomatal closure (Else et al., 1995) and is also involved in the development of aerenchyma in roots (Pan et al., 2021).
Absorption of mineral nutrients
The severe energy deficit of roots due to O2 deficiency induces an irregular functioning of these organs, resulting in deficient absorption and transport and an insufficient supply of the plant with nutrients and water (Zeng et al., 2014; Lambers & Oliveira, 2019). Suppression of the root colonization by mycorrhizal fungi in waterlogged soils contributes to reduced nutrient uptake (Kozlowski & Pallardy, 1997), but in peach seedlings an arbuscular mycorrhizal (AM) infection provides limited tolerance to waterlogging and flooding, possibly due to increased plant nutrition (Rutto et al., 2002). However, waterlogging favors the risk of nutrients leaching to deeper layers of the soil (Friedrich & Fischer, 2000).
Waterlogging impairs the absorption of ions due to its impact on soil conditions as well as the response of plants to nutrient uptake that is less affected in species tolerant to waterlogging (Pallardy, 2008). Especially for species intolerant to waterlogging, the uptake of N, P, and K is decreased by the decrease in energy due to anaerobiosis, while the absorption of Ca and Mg is less affected, and that of Fe and Mn increases due to its reduction to Fe2+ and Mn2+, respectively (Kozlowski & Pallardy, 1997). Regardless of the increase in the levels of Fe and Mn in the rhizosphere, their uptake by the roots is reduced due to slower growth of the flooded plants (Pallardy, 2008). In contrast, 'Peach' mango (Mangifera indica) trees increase their photosynthetic rate by increasing Fe and Mn levels in calcareous soil, when the 10 to 20 d of waterlogging was pushed to higher levels than in the previous stage of waterlogging (Schaffer et al, 2009).
Waterlogging increases soil N loss due to denitrification, nitrate leaching, and runoff, reducing the N mineralization rate in the soil (Kaur et al., 2020). Foliar applications of N could reduce the detrimental effect of waterlogging in lulo (naranjilla) (Solanum quitoense) plants (Flórez-Velasco et al., 2015). The foliar application of nutrients can compensate for the inhibited absorption of mineral nutrients by the roots. In addition, the foliar application of phytohormones can compensate their reduced synthesis due to the limited function of the roots, as is the case of cytokinins and gib-berellins (Moreno & Fischer, 2014).
Interaction with other climatic factors
In flooded pitanga (Eugenia uniflora), a pre-acclimatization to high solar radiation (44 mol m-2 d-1), for 55 d, decreased the photosynthetic rate and the accumulation of dry weight compared to those only exposed to a radiation of12 mol m-2 d-1 (Mielke and Schaffer, 2010). Additionally, lulo (naranjilla) plants under shade (56%) tolerated waterlogging better than those without shade (Sánchez-Reinoso et al., 2019).
However, low solar radiation arising during periods of intense and prolonged rains that flood the plantations can reduce photosynthesis and further affect plant growth (Pucciariello & Perata, 2012). For the chonto tomato (Ly-copersicon esculentum), 56% shading combined with 12 d waterlogging reduced biomass accumulation in the plant more than shade or waterlogging alone (Baracaldo et al., 2014). Two or more combined abiotic stresses can affect physiology and productivity of plants that are different from effects of individual stress (Taiz et al., 2017).
High temperatures aggravate the effects of waterlogging and increase plant mortality due to decreased solubility of O2 and CO 2 in warm flooded water (Panda & Barik, 2021). Citrus roots soaked for 3 d at 30-35°C die, however, at temperatures below 15°C can survive for several months (Orduz-Rodríguez, 2012). The abscission of leaves, flowers, and fruits due to waterlogging is more accentuated when the temperature of the soil and water increase above tolerance limits (Fischer et al., 2016).
Adaptation and tolerance mechanisms
Tolerance to waterlogging depends on several factors; for example, in fruit species, the previous stress of the plant (e.g., in too cold or dry climates), the fruit load, the air temperature (warmer ones being more harmful), the type of soil, and the depth of the flood and its duration influence this tolerance (Crane et al., 2020).
Plants have developed different morphological, physiological, and biochemical adaptations to survive O2 deficiency stress, including metabolic acclimation and signaling networks, so that plants can resist or escape low-O2 environments that disturb their metabolism and growth (Xie et al., 2021).
Parent et al. (2008) consider that species tolerant to waterlogging are generally those that are able to continue their energetic state through fermentation. As a short-term response, there is an increase in the antioxidant defense system as a reaction to the increase in ROS; and this increases the level of antioxidant enzymes serving as a parameter for tolerance to waterlogging stress (Jiménez et al., 2012). Thus, in apples the activity of superoxide dismutase (SOD), per-oxidase (POD), and glutathione reductase (GR) increases during hypoxic conditions (Bai et al., 2010).
After the metabolic adjustment, the plant generates morphological changes, such as formation of aerenchyma in roots of some species to survive long periods of flooding (Jiménez et al., 2012). The formation of an aerenchyma, induced by waterlogging, consists of interconnected intercellular spaces caused by the programmed death of cortical cells that can promote gas exchange with the aerial part of the plant and improve adaptation to hypoxic conditions (Rankenberg et al., 2021) due to the significant increase in the energy status of the plants (Jiménez et al., 2012). In the submerged soils, ethylene induces the formation of aerenchyma in the mature parts of the root (Pedersen et al., 2020) within its fully developed cortex (Rankenberg et al., 2021). Ethylene prevents the elongation of the roots and leads to the formation of an aerenchyma (Lambers & Oliveira, 2019).
Above all, in trees with deep roots that grow in humid soils the O2 supply is greatly limited. This is why some species survive through anaerobic metabolism (by fermentation) or through the formation of pneumatophores that allow the movement of O2 to the roots (Taiz et al., 2017). In addition, plants that show a certain tolerance to waterlogging can induce formation of adventitious roots that contain aerenchyma for the diffusion of O2 from the aerial parts of the plant (Jackson & Colmer, 2005) and in waterlogged chonto tomato (Baracaldo et al., 2014). Generally, endogenous auxin is responsible for the formation of adventitious roots (Lambers & Oliveira, 2019). These new roots can compensate for the loss of the ability to absorb water and nutrients from the decayed root system (Kozlowski & Pallardy, 1997). In many cases, the adventitious roots are formed near the base of the stem or in the area of numerous lenticels (Parent et al., 2008).
Apart from adventitious root formation, a rapid elongation of the stem is one of the most important morphological adaptations in highly flooded plants (Xie et al., 2021). Also, in short-term flooding (2-6 d) in tree tomato (Solanum betaceum), the plants react with greater stem elongation; however, they are accompanied by a loss of plant biomass (Betancourt-Osorio et al., 2016).
Several mango genotypes react to waterlogging with the formation of hypertrophied lenticels that facilitate O2 diffusion (Schaffer et al., 2009) and the release of toxic substances produced by anaerobic metabolism (Parent et al, 2008). Because of that, Zeng et al. (2014) highlight the importance of the supply of internal O2 from the tree trunk that is crucial for the functioning of the roots in an anoxic environment.
In waterlogged plants, the induction of epinasty can be observed that has the effect of decreasing foliar transpiration, generating protection against direct sun and heat to the leaves (Schaffer et al., 2006).
Some measures to relieve stress from waterlogging
In case of fruit trees that need to be grafted, the selection of more tolerant rootstocks reduces hypoxia stress to a minimum (Schwarz et al., 2010); the use of rootstocks from other species, such as plum (Prunus domestica) and for some peach and apricot (Prunus armeniaca) varieties has led to greater resistance to this problem (Orazem et al., 2011; Reig et al, 2018). Likewise, in tropical and subtropical fruit trees, such as citrus, the Citrus macrophylla rootstock shows greater tolerance to waterlogging than 'Cleopatra' (C. reshni) (Pérez-Jiménez & Pérez-Tornero, 2021), just as the 'DusaTM' rootstock is more resistant than the 'Duke 7' avocado (Persea americana) (Reeksting et al., 2014). The type of rootstock plays an important role in the adaptation to waterlogging; guava plants propagated by seedlings are more tolerant than those propagated by shoot layering (Kongsri et al., 2020).
When waterlogging ends, pruning the branches can restore the balance of the shoot/root ratio in the tree, especially if there are damaged roots; thus, pruned avocado trees recover more quickly from hypoxic stress, compared to those not pruned (Sanclemente et al., 2014). In addition, the reduced leaf area limits transpiration, which is difficult in plants with damaged roots.
The redox potential of flooded soil can be improved with the application of solid fertilizer such as CaO or MgO. For papaya (Caricapapaya), adding 5 g of magnesium peroxide (MgO) to the potting medium prior flooding increases the leaf area and the total dry weight of the flooded plants compared to the non-flooded ones because of the oxygenation of the soil (Thani et al., 2016). This improves the redox potential, an indirect indication of O2 content (Liu & Porterfield, 2014).
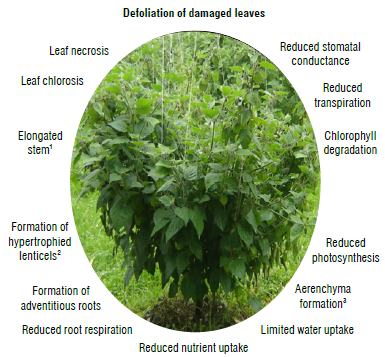
1 Under flooded conditions;
2 in some mango varieties; 3 in some species.
FIGURE 1 Schematic diagram of the effect of waterlogging stress on important processes in tropical and subtropical fruit crops, including adaptive responses. Modified from Sathi, K. S., Masud, A. A. C., Anee, T. I., Rahman, K., Ahmed, N., & Hasanuzzaman, M. (2022). Soybean plants under waterlogging stress: responses and adaptation mechanisms. In M. Hasanuzzaman, G. J. Ahammed, & K. Nahar (Eds.), Managing plant production under changing environment (pp. 103-134). (With permission from Springer Nature Singapore).
Similarly, mycorrhizal colonization of roots can increase tolerance to waterlogging because they promote growth and biomass of plants by improving nutritional conditions and potential adjustment (Tuheteru & Wu, 2017). Flooded purple passionfruit (Passiflora edulis f. edulis) plants inoculated with a mixture of mycorrhizae (Glomus caledonium, G. etunicatum, Gigaspora margarita, and Scutellospora sp.) showed an increased retention of leaves and increased proline and chlorophyll content in leaves and longer maintenance of the foliar content of N and P (Chebet et al., 2020). These authors supposed that the increasing dry and fresh root weights contributed to a higher root health of mycorrhized plants favoring the uptake of mineral nutrients.
Foliar applications of glycine betaine (GB) or hydrogen peroxide (H2O2) provides plants with a greater tolerance to waterlogged conditions, favoring their acclimatization. Cape gooseberry (Physalis peruviana) flooded for 6 d with applications of 100 mM GB or H2O2 show better responses in terms of growth and physiology (stomatal conductance, maximum photochemical efficiency of PSII [F v /F m ], leaf water potential, relative water content, chlorophyll content, and net photosynthesis) (Castro-Duque et al., 2020). In general, chlorophyll fluorescence is a fast and easy technique to measure the effect of waterlogging stress (Flórez-Velasco et al., 2015; Sánchez-Reinoso et al., 2019; León-Burgos et al., 2022).
Fruit trees adapt better to conditions of abundant rainfall when they are planted in places where floods have not historically occurred, such as on sloping land (Fig. 2). Other measures include avoiding planting crops near bodies of water (lakes, rivers, etc.), avoiding unevenness in planting lots, planting trees in raised beds (Fig. 3) with deep subsoil, and installing drainage (Moreno & Fischer, 2014; Kaur et al., 2020).

FIGURE 2 Flooding of Chicamocha river near Duitama (Colombia), with 'Anna' apple (Malus domestica) plantation on slopes. Photo: G. Fischer.

FIGURE 3 Raised beds of papaya (Carica papaya) plantation prevent waterlogging effects on the roots, La Union (Valle, Colombia). Photo: G. Fischer.
In soils that experience waterlogging and flooding, the long-term use of cover plants improves the soil structure and reduces its compaction by increasing the water infiltration rate (Blanco-Canqui et al., 2015).
Waterlogging and plant diseases
Waterlogged soil, especially in the case of standing water, provides an ideal culture medium for many fungal and bacterial pathogens (Friedrich & Fischer, 2000). This potent combined stress weakens plants, especially the root system that becomes more susceptible to diseases, particularly in avocado, papaya, lychee (Litchi chinensis), and pineapple (Ananas comosus) (Paull &Duarte, 2012).
Stagnant water leads to root rot in avocado by Phytoph-thora cinnamomi (Reeksting et al., 2014), in pineapple by Phytophthora spp. (Fischer, 2012) (Fig. 4), in papaya because of Pythium aphanidermatum (Koul et al., 2022), in banana by Fusarium oxysporum f. sp. cubense (Bolaños, 2019), and in cape gooseberry by F. oxysporum (Villareal-Navarrete et al., 2017; Fischer & Melgarejo, 2020). Cape gooseberry infected with F. oxysporum f. sp.physalis shows low acclimatization for periods that exceed 6 d of flooding (Arias et al., 2019). One measure to alleviate disease stress in waterlogged soils is the use of rootstocks that are more resistant to these pathogens.
Some examples of studies on fruit trees
Crane et al. (2020) defined three flood tolerance groups of fruit trees: 1) Tolerant - trees can survive high water table and flooded conditions up to a few weeks, but these conditions can reduce growth and production. In this group these authors include: guava (Psidium guajava), sapodilla (Manilkara sapota), caimito (Pouteria caimito), coconut (Cocos nucifera) and grafted citrus (Citrus spp.); 2) Moderately tolerant trees can survive several days of waterlogged or flooded soil conditions, but these conditions can reduce growth and production (e.g. lychee (Litchi chinensis), longan (Dimocarpus longan), 'Tahiti' lime (Citrus x latifolia), canistel (Pouteria campechiana), mango (Mangifera indica), carambola (Averrhoa carambola) and banana (Musa x paradisiaca); 3) Intolerant trees do not tolerate waterlogged or flooded soil conditions that after one or a few days of these conditions can heavily damage or kill the trees (e.g. avocado (Persea americana), papaya (Carica papaya), mamey sapote (Pouteria sapota), sugar apple (Annona squamosa), atemoya (Annona x atemoya), passion fruits (Passiflora spp.) and jackfruit (Artocarpus heterophyllus). Crane et al. (2020) underline that the first and second group of fruit crops mentioned above can be damaged or killed by root diseases.
Peach and avocado do not tolerate waterlogging and need about 15% O2 in the soil solution for survival compared to pear (Pyrus communis) and apple that resist up to a 5% O2 in the soil solution (Fischer & Orduz-Rodríguez, 2012). Passion fruit species, in particular yellow passion fruit (P. edulis f. flavicarpa) and purple passion fruit (Tab. 1), can tolerate waterlogging conditions for a few days (Fischer & Miranda, 2021), although these findings are probably due to the distinct experimental methods used by the researchers.
Paull and Duarte (2012) also include pineapple as a very sensitive crop to waterlogging. Saavedra et al. (2012) attribute the intolerance of avocado to waterlogging because of a lack of absorbing root hairs. Moreno and Fischer (2014) classify species of the genus Prunus such as cherry (P. avium) and plum (P. domestica and P. salicina) as intermediate tolerant to waterlogging, as well as citrus (Citrus spp.). In the case of bananas, Bolaños (2019) distinguishes between flooding with circulating water that the plants can tolerate up to 72 h, while under non-circulating flooding they only tolerate 24-48 h (Robinson & Galán-Sauco, 2010).
For several fruit trees of tropical and subtropical origin (Tab. 1), waterlogging reduces stomatal conductance, thus affecting the photosynthetic rate, leading to lower leaf area and biomass production in the plant organs (Sanclemente et al., 2014). Increase in chlorosis and necrosis of the leaves leads to a decrease in the chlorophyll content: cape gooseberry shows a 51% reduction in the chlorophyll index for 8 d of waterlogging, observed after 50 d (Aldana et al., 2014). This premature leaf senescence is attributed to the reallocation of mobile elements in the phloem as N, P, and K to younger leaves because of the hypoxic root conditions (Kläring & Zude, 2009).
TABLE 1 Effect of waterlogging on the growth and physiology of some tropical and subtropical fruit trees.
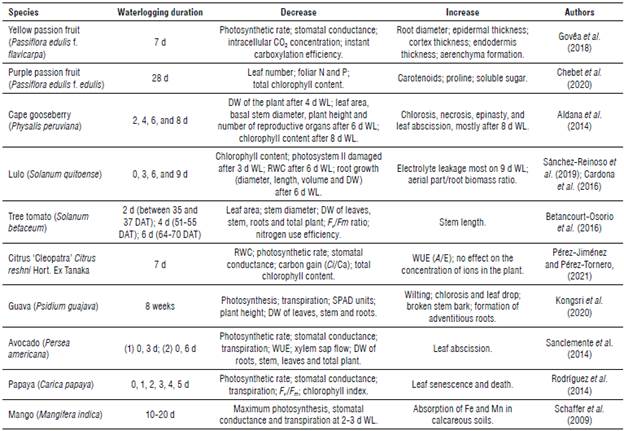
DW: dry weight; WL: waterlogging; RWC: relative water content; WUE (A/E): water use efficiency; F v /F m : maximum photochemical efficiency of photosystem II; Ci/Ca: carbon gain; DAT: days after transplanting.
Measurements of chlorophyll fluorescence in lulo (naranjilla) shows damage at the level of photosystem II from 3 d of waterlogging; the drastic decrease in the maximum efficiency of photosystem II (PSII) (F v /F m ) is especially evident in plants waterloged for 9 d (Sánchez-Reinoso et al., 2019). In papaya, a decrease in the F v /F m values occurs after only 2 d of flooding (Rodríguez et al., 2014). Due to its practical use, Moreno et al. (2019) recommended the use of the maximum photosynthetic efficiency of PSII and the stability of the cell membranes as markers for tolerance under waterlogging conditions in trees. The increase in chlorosis and necrosis in the leaves leads to a decrease in the chlorophyll content, probably, caused by the deficient absorption and translocation of mineral nutrients and water by the roots (Fischer et al., 2016).
In general, the results presented in Table 1 show that the tolerance of tropical and subtropical fruit trees to flooding conditions depends on the species, with Solanaceae being among the most affected. These include cape gooseberry (Aldana et al., 2014) (Fig. 5) or naranjilla (Sánchez-Reinoso et al., 2019), which tolerate less than 6 d of flooding. Guava is a very waterlogging-tolerant species (Crane et al., 2020); its seedlings tolerate waterlogging for 8 weeks without dying, and they react with the formation of adventitious roots after 5 weeks (Kongsri et al., 2020).
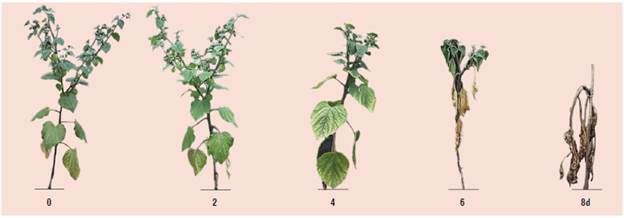
FIGURE 5 Symptomatology of cape gooseberry plants through 0, 2, 4, 6, and 8 d of waterlogging, at 50 d after the beginning of waterlogging (modified from Aldana et al. (2014), with permission of Revista de la Academia Colombiana de Ciencias Básicas Exactas, Físicas y Naturales).
Basso et al. (2019) classify the yellow passion fruit as a species that does not tolerate more than 4 d of waterlogging due to the irreversible effects on its growth and physiology. Govêa et al. (2018) find that the waterlogged yellow passion fruit plants had a certain tolerance due to an increase in the diameter of the roots, epidermis, cortex, and endodermis, and the formation of aerenchyma compared to non-waterlogged plants. Faria et al. (2020) classify passion fruit plants as moderately susceptible to excess water in their root system.
No studies have been carried out on the effect of waterlogging on fruit trees from the tropical and subtropical zone growing in the highlands; these will be greatly affected by climate change. This situation is mentioned by Fischer and Parra-Coronado (2020) and Fischer et al. (2020) for feijoa (Acca sellowiana),Fischer et al. (2021) for sweet cucumber (S. muricatum), and by Fischer and Miranda (2021) for banana passion fruit (P. tripartita var. mollissima).
Conclusions
Because of the effects of climate change and especially global warming, waterlogging and flooding incidents in tropical and subtropical fruit crops have increased in frequency and are unpredictable. As a result of the anaerobic conditions in the rhizosphere, phytotoxic conditions increase from the accumulation of reduced ions and products of anaerobic organisms, offering an ideal culture medium for many fungal and bacterial pathogens.
Waterlogging leads to depletion of O2 in the soil with detrimental impacts on root growth and functions and ultimately on the entire plant and its physiology. A decrease in the photosynthetic rate is especially considerable, due to reduced intact leaf areas as a result of chlorosis, necrosis and leaf drop, stomatal closure, and chlorophyll degradation.
Plants have developed different morphological, physiological, and biochemical adaptations to survive stress due to O2 deficiency in soil. Some fruit trees form an aerenchyma for the diffusion of O2 from the aerial parts of the plant in order to survive long periods of flooding, in addition to the induction of aerenchyma-containing adventitious roots. A rapid elongation of the stem is one of the most important morphological adaptations of some flooded plants, likewise the formation of hypertrophied lenticels in mango varieties.
Measures for better adaptation of tropical fruit crops to this climatic impact include adaptation of the area of cultivation, selection of varieties and rootstocks more tolerant to hypoxia stress, pruning to reestablish the balance of the aerial part/root ratio in trees, and foliar applications of glycine betaine or H2O2. Mycorrhizal colonization in the roots can increase tolerance to waterlogging and the application of fertilizers such as CaO or MgO can improve the redox potential of flooded soil.
Results of studies on the effect of waterlogging on the growth and physiology of tropical and subtropical fruit trees show that guava and coconut are the most tolerant species resisting hypoxia stress during some weeks.















