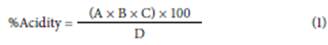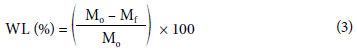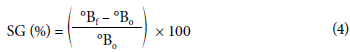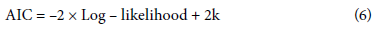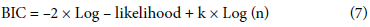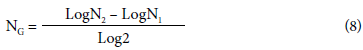Introduction
Most foods that contain probiotics are derived from milk (Vinderola et al., 2017). This creates a big problem for some consumers since it involves the consumption of allergens and lactose. In addition, it implies the consumption of products of animal origin that are not suitable for a vegetarian or vegan diet and have a short shelf life (Makinen et al., 2016). Because of this the demand for non-dairy probiotic products has grown because of an increased incidence of dietary restrictions on dairy products for reasons including lactose intolerance, allergic reactions to milk proteins, and veganism (Kumar et al., 2015; Neffe-Skocinska et al., 2018).
Probiotics are defined as live microorganisms that, when administered in sufficient amounts, confer a health benefit on the consumer (Hill et al., 2014). Saccharomyces boulardii is included among the microorganisms with probiotic effect. This microorganism can remain viable and active so that when ingested it can give pharmacodynamic effects similar to the physiological effects of normal intestinal flora (Peña, 2007; Zamora Vega et al., 2015); it also acts as a transporter, releasing enzymes, proteins, and trophic factors during its intestinal transit, improving the host's immune defenses, digestion, and nutrient absorption. So, this yeast is an important source for probiotic products (Mejía et al., 2016; Sen & Mansell, 2020). Its probiotic activity has been linked to multiple pathways, including enhancement of gut barrier function, competitive pathogen exclusion, antimicrobial peptide production, and immunomodulatory and nutritional effects (Zhang et al., 2021). This probiotic strain helps in maintaining the balance of intestinal flora by stimulating the production of lactic acid and acetic acid, lowering intestinal pH, and preventing the proliferation of pathogenic bacteria (Li, Xia et al., 2021; Li, Zhu et al., 2021).
Fruits and vegetables are an essential part of the human diet, since they are a source of bioactive compounds such as vitamins, minerals, phytosterols, dietary fiber, etc. Generally, fruits and vegetables are marketed fresh. However, their shelf life is limited by metabolic activity, and they are susceptible to mechanical damage and the presence of microorganisms that accelerate their senescence and death (Yousuf et al., 2018; Al-Tayyar et al., 2020). Osmotic dehydration is a technique used to reduce the water content and to include solutes in fruits and vegetables by immersion in an osmotic solution. This technique has been widely used in the food industry to produce dehydrated fruits with a longer shelf life and nutritional value (Yousuf et al., 2018).
The apple fruit matrix has been proven to be highly applicable for probiotics, possibly due to its high porosity and, therefore, the easy incorporation of probiotics. Espírito Santo et al. (2012) attribute this to the cellulose content in apples that is not digested and could serve as a protective matrix for probiotics through the intestinal tract (Kourkoutas et al., 2006). Rêgo et al. (2013) demonstrate the compliance of apple as a fruit matrix for probiotic survival over time. They studied hot-air-dried apple cubes with Lactobacillusplantarum incorporated during 65 d of storage and found a loss of viability of 1 log CFU/g.
Gupta and Garg (2009) suggest a dose of 5 billion colony forming units (CFU) for at least 5 d (5 x 109 CFU/d) to produce health benefits. Probiotics may be available in foods and dietary supplements (capsules, tablets, and powders). Also, to improve probiotic survival, the food should be dehydrated by lyophilization instead of hot air (Betoret et al., 2003; Rascón et al., 2018). Another methodology that is increasingly of interest in the impregnation of probiotics is osmotic dehydration. This consists of submerging the food in a hypertonic solution that contains microbial cells. The water migrates from the food to the solution, partially dehydrating it; and the solutes, including the probiotics, migrate towards the food (Rascón et al., 2018).
In a study carried out on plantain impregnated with Lactobacillus rhamnosus, the process was successful, maintaining levels of 107 CFU/g (Huerta-Vera et al., 2017). Using 50% w/w hypertonic sucrose solutions, Rascón et al. (2018) impregnated L. rhamnosus in banana slices by osmotic dehydration to subsequently freeze-dry them. The survival kinetics of the microorganism showed that its viability decreased significantly when water activity (aw) exceeded values of 0.327.
The purpose of this study was to evaluate how temperature and concentration influence the stability of probiotics (specifically Saccharomyces boulardii) during the osmotic dehydration of Granny Smith apple (Malus domestica) cubes.
Materials and methods
Raw material
Granny Smith apples were purchased at the local market in the province of San Román, department of Puno (Peru) and stored at 4°C. We washed them for 5 min in an aqueous solution of active chlorine at a concentration of7500 mg L-1 and then cut them into cubes of 1 cm square. Freeze-dried strains of S. boulardii Hansen CBS 5926 from Mexico were inoculated into the solutions.
Maturity index
To determine the maturity index in apples, we measured °Brix and acidity. According to the AOAC (2005) method, we determined the acidity by preparing a 0.1N NaOH solution; then we weighed 10 g of apple pulp and added 50 ml of distilled water. The mixture was vigorously shaken for a few minutes, and we collected an aliquot of 25 ml of the solution. Once filtered, we added 3 drops of phenolphthalein. We titrated the solution with a standard 0.1 N NaOH solution until it reached the equivalence point by changing color to light pink. We used the volume spent to calculate the acidity percentage (Eq. 1). We determined Brix with an ATAGO range refractometer (0-40°Brix).
where A represents the volume of NaOH spent, B signifies the normality of NaOH (0.097 meq ml-1), C denotes the equivalent weight expressed in grams of the predominant acid in the fruits (citric acid 0.064 g meq-1; malic acid 0.067 g meq-1), and D stands for the weight in grams of the sample used.
The maturity index (MI) was determined with the following formula:
Osmotic dehydration and inoculation of the strain
We prepared aqueous osmotic solutions with sucrose at different concentrations (40°Brix, 50°Brix, and 60°Brix). The apple cubes were immersed in 500 ml beakers with 200 ml of the prepared osmotic solution at three different temperatures (37°C, 42°C, and 47°C), and 250 mg of S. bou-lardii were immediately added. We applied treatments by shaking at 350 rpm. Each apple sample was removed every 10 min up to a total time of 80 min to conduct weight measurements on an OHAUS analytical balance and measure the soluble solids content in the liquid phase (Brix) using an ATAGO range refractometer (0-85°Brix). We conducted all the experiments in triplicate.
Weight loss and solid gain
During osmotic dehydration, we monitored mass transfer by the time variation in solid gains and weight loss (Della Rocca & Mascheroni, 2011; Wais, 2011).
Equation 3 was used to calculate weight loss.
where WL (%) is the percentage of weight loss, Mo represents the initial weight of the sample in grams, and Mf is the weight of the sample treated at time t.
The solid gains were calculated as indicated by Equation 4.
where SG (%) were the solid gains percentages, °Bf were the Brix degrees of the osmodehydrated sample and °Bo were the Brix degrees of the fresh sample.
Microbiological kinetics
To assess the microbiological growth kinetics in the polynomial model, we employed several key statistical metrics. To gauge the model's fitness for survival, doubling time, and the number of generations, we utilized the coefficient of determination (R2). We computed this coefficient using the following equation:
where SSE (Sum of Squared Errors) represented the sum of the squares of differences between actual observations and model predictions, and SST (Total Sum of Squares) was the sum of the squares of differences between actual observations and their mean.
Additionally, to evaluate the model's goodness of fit in terms of complexity, we employed the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC). We calculated AIC using the following equation:
where log-likelihood was the logarithmic likelihood function of the model and k was the number of estimated parameters in the model.
BIC was computed as follows:
where log-likelihood was the logarithmic likelihood function of the model, k was the number of estimated parameters, and n was the number of observations in the data.
We applied these statistical metrics in the study's methodology to assess the goodness of fit of the polynomial model and its ability to explain the observed data.
Survival, doubling time, and number of generations
To determine cell survival, we used a 10-1 dilution in 1% saline solution, and then Petri dishes were inoculated with 0.1 ml of Sabouraud Dextrose Agar and dispersed with a Drigalski spatula; we used a convection incubator at 27°C for 48 h; the readings were expressed in colony-forming units per gram (CFU/g).
To calculate the number of generations, we applied the following equation:
where NG was the number of generations, N2 was the concentration of cells at time 2 and N[ was the concentration of cells at time 1, expressed in colony-forming units per gram (CFU/g).
The doubling time was calculated with the following equation:
where TD was the doubling time, tf was the final incubation time, and tt was the initial incubation time in minutes.
Results and discussion
Osmotic dehydration
In Figure 1, the treatments with the highest percentage of weight loss were the treatments 50°B47°C, followed by 50°B37°C. In contrast, the treatment 60°B42°C obtained an unfavorable result. All other treatments had a positive slope indicating that weight loss was continuous over time.
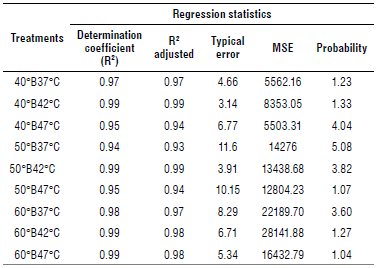
TABLE 1 Statistics of the linear regression of the percentage of weight loss in apple cubes inoculated with Saccharomyces boulardii
Note: In each treatment, В corresponds to degrees Brixinthe medium followed by the treatment temperature. MSE-Mean Square Error.
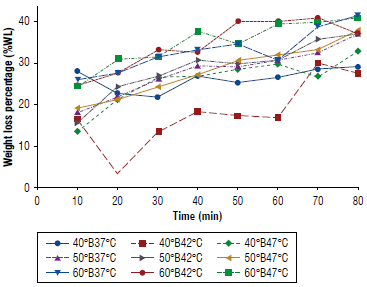
FIGURE 1 Weight loss percentage (%WL) as a function of time of apple cubes inoculated with Saccharomyces boulardii at different concentrations and temperatures.
Figure 2 shows that the gain of solids increased as the treatment time increased. This was probably due to the incorporation of soluble solids in the fruit. Also, the rate of gain of solids increased as the concentration increased.
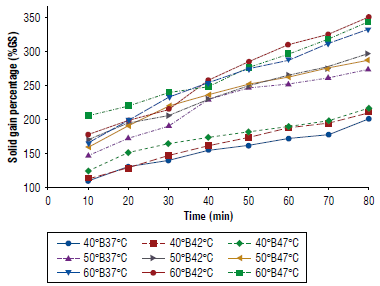
FIGURE 2 Solid gain percentage (%GS) as a function of time in apple cubes inoculated with Saccharomyces boulardii at different concentrations and temperatures.
The statistical analysis of the regression (Table 1) revealed that 50°B47°C was the treatment with the best fit with an R2of 0.98 in contrast to the others. Likewise, it showed the highest value in the R2 adjusted (0.98) related to the typical error. The same treatment showed the smallest value of typical error (0.91). On the other hand, all the treatments were significant with a P-value less than 0.05. However, two had higher values, 40°B42°C with 0.21 and 50°B42°C with 4.60.
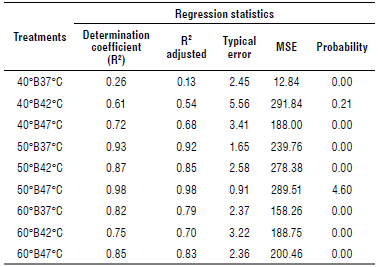
Table 2 presents the results of the statistical regression analyzes, where all the treatments had a good adjustment (R2>0.93) ranking between 0.94 and 0.99. The error 40°B42°C and 50°B42°C had the smallest values of all the treatments.
Microbiological kinetics
The microbiological kinetics of each of the treatments revealed intriguing findings. Specifically, S. boulardii experienced a significant increase in its development at a temperature of 42°C across all evaluated concentrations. This strongly suggested that this temperature represented the optimal condition for cellular reproduction of this microorganism. This discovery holds significant relevance as it provided valuable insights into the ideal conditions for the cultivation and stability of S. boulardii in osmotic dehydration. Furthermore, when analyzing the kinetic curves in 60°Brix media, we noted a distinct behavior. These curves exhibited significantly lower values compared to the other two graphs. This phenomenon could be associated with the influence of sugar concentration in the growth medium on the activity and growth of microorganisms. These results open new avenues of research to better understand how environmental variables, such as temperature and nutrient concentration, impact the growth kinetics of biotechnologi-cally relevant microorganisms. This, in turn, could lead to improvements in the production and application of S. boulardii in apple cubes.
Survival of Saccharomyces boulardii
The survival of S. boulardii during osmotic dehydration exhibited divergent responses across the various treatments. In certain instances, such as in the case of 60°B47°C and 40°B47°C, we observed limited development with values of 1.75 and 1.72 CFU/g. Conversely, two treatments yielded the highest number of generations: 50°B37°C with 9.32 CFU/g and 60°B37°C with 8.08 CFU/g. Furthermore, these two treatments demonstrated the shortest doubling times, indicating a more rapid increase in biomass.
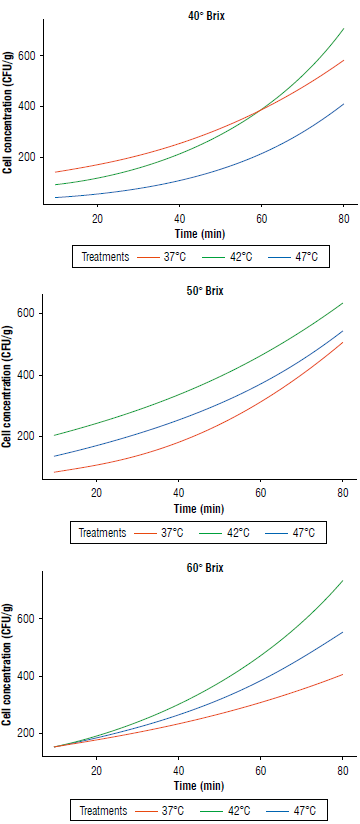
FIGURE 3 Microbiological kinetics of Saccharomyces boulardii in apple cubes at different concentrations of solids and temperatures.
Specifically, 50°B37°C had a doubling time of 7.50 min, whereas 60°B37°C had a doubling time of 8.69 min (Tab. 3). These findings underscored the significant influence of temperature and solute concentration conditions on the survival and growth of S. boulardii during osmotic dehydration. Treatments conducted at lower temperatures, such as 50°B37°C, facilitated greater bacterial growth and more abundant bio mass compared to treatments conducted at higher temperatures, such as 60°B47°C. These results emphasized the critical importance of judiciously selecting osmotic dehydration conditions to achieve desired outcomes in terms of microbial survival and growth.
TABLE 3 Viability of S. boulardii in apple cubes inoculated during osmotic dehydration at different concentrations and temperatures.
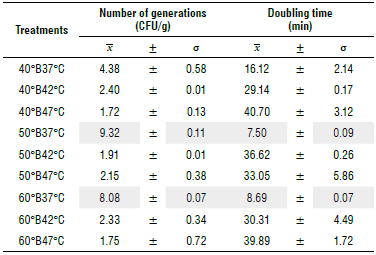
Note: All means are expressed as mean ± σ (n = 3). In each treatment, В corresponds to degrees Brix In the medium followed by the treatment temperature.
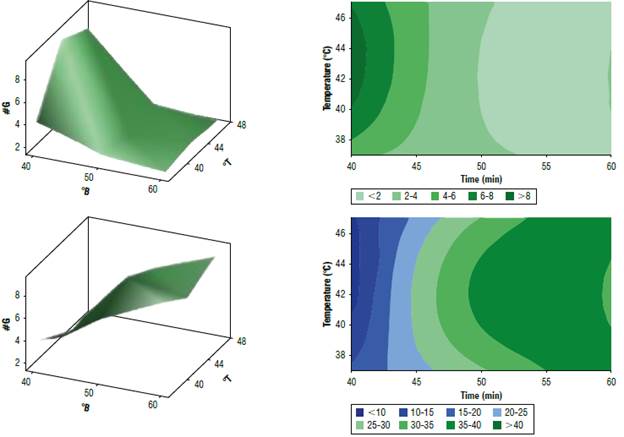
FIGURE 4 Response surface and contour plots for survival of S. bou pressed in CFU/g, °B is °Brix, T is the temperature in C° and TD is the doubling time in minutes
In the analysis of the response surface graph for the number of generations, increasing the total solids concentrations (°Brix) had a noticeable impact on biomass concentration. This impact varied with temperature. Initially, as we increased the solids concentration, the biomass concentration also increased, and this relationship held true up to a certain point. However, beyond that point, there was a decline in biomass concentration. This trend is clearly illustrated in the cont our graph, where the green region represents the range of conditions that result in the highest biomass concentrations.
Likewise, when examining the response surface for doubling time, at higher concentrations of solids and temperatures, the rate of cell doubling increased. The contour graph further corroborated this observation. It highlighted the blue area that signified those lower solid concentrations (specifically 40 and 45°Brix) lead to the shortest doubling times within the temperature range of 40°C to 47°C. This means that under certain conditions, cells doubled more rapidly. This could be crucial information for optimizing the process.
Discussion
Cui et al. (2018) evaluate weight loss during osmotic dehydration of pears in solutions of sucrose and sodium chloride at different concentrations. Their results show that weight loss increases with the concentration of the solution and with dehydration time, agreeing with the data shown in Figure 1. This also agrees with the findings of Ayala-Aponte et al. (2010) in mangoes with sucrose solutions, where weight loss increased with the concentration of the solution and with dehydration time.
Parra-Palacios (2020) mentions that the increase in weight loss in pineapple slices shows that when the concentration and temperature increase there is a 19.50% weight loss.
Giraldo et al. (2005) point out that the weight loss in mango at different concentrations is due to the amount of water transferred from the mango to the osmotic medium that is greater than the quantity of soluble solids that migrate from the hypertonic medium. Likewise, Della Rocca and Mascheroni (2011) argue that temperature is one of the most significant variables since it will modify the kinetics in the osmotic dehydration process, hence weight loss. Weight loss is more affected than a gain in soluble solids; because of the use of high temperatures, the syrup solute cannot easily diffuse.
In Figure 2, the results of a gain of solids with respect to time are shown. It is possible to observe that weight loss increases as the treatment time increases. This is probably due to the incorporation of soluble solids in the fruit; and, also, the rate of solid gains increased as the concentration of the osmotic solution and the temperature increased. This agrees with what is found by Ochoa and Ayala (2009) who state that in slices of yacón an increase in solids is proportional to the concentration and temperature. Huerta-Vera (2021) reveals that at higher concentrations of the osmotic solution, the solid gain increased during osmotic dehydration in chayote slices, while an increase in the temperature of the osmotic medium also causes an increase in an increase in solids of chayote. Likewise, the results Arias et al. (2017) indicate that as the temperature of the osmotic solution increases, there is a greater alteration of the cellular structure, which favors the entry of solutes into the interior of the food. To limit the impregnation, it is convenient to use high solute concentrations and short osmotic dehydration times (Della Rocca & Mascheroni, 2011).
The survival of S. boulardii under osmotic dehydration conditions in the different treatments showed that under adverse conditions these could be inhibited, generating an increase in the biomass concentration (Tab. 3, Fig. 4). This is corroborated by Rascón et al. (2018) who affirm that the addition of Lactobacillus rhamnosus to banana slices causes an inhibition after 300 min, achieving a concentration of 9.40 ± 0.23 log 10 CFU/ml. Likewise, Rodrigues et al. (2018) agree that a prolonged time for Lactobacillus casei in apples dried at 60°C generates a decrease in biomass, which shows that the treatments carried out at low temperatures (37°C and 42°C) do not exceed the adverse conditions for the normal development of the biomass.
Conclusions
In this study, microbiological growth kinetics were assessed under osmotic dehydration conditions. We observed that treatments 50°B47°C and 50°B37°C exhibited the highest weight loss and displayed an excellent fit in the regression analysis, with R2 values of 0.983 and 0.980. Furthermore, they stood out for their low typical errors (0.912).
Regarding microbiological kinetics, 42°C represented the optimal temperature for the growth of S. boulardii across all evaluated concentrations. Additionally, sugar concentrations significantly influenced the kinetics, resulting in reduced growth at 60°Brix.
Survival of S. boulardii during osmotic dehydration varied among treatments, with 50°B37°C and 60°B37°C demonstrating a higher number of generations and shorter doubling times. These findings underscored the critical influence of temperature and solute concentrations on microbial survival and growth during osmotic dehydration.
In summary, this study furnished valuable insights into the optimal conditions for the growth and survival of S. boulardii during osmotic dehydration. These discoveries hold significant implications for the production and application of S. boulardii in apple cube processing and pave the way for further research into environmental variables affecting microbial kinetics.