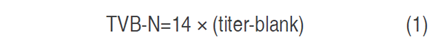Lignocellulosic materials such as straws, stalks, and shells, offer a plentiful and renewable carbon resource for diverse fermentation processes, ensuring long-term sustainability (Singh et al. 2012). Growing interest in harnessing lignocellulosic materials from agricultural and domestic sources has led to significant economic and environmental benefits. These include reducing land usage for waste disposal and valorizing these wastes by utilizing them as raw materials for various bio-production processes (Singh et al. 2012).
Wheat straw, comprising over 80% of total domestic agricultural residues, is widely regarded as an ideal biomass feedstock because of its relatively low cost and the high volume of lignocellulose present in the biomass (Kadam and McMillan 2003). The current availability of wheat straw is estimated at 80 million dry tons per year (USDA 2003), a majority of which could be available to bio-production plants in the near term. Its exploitation and transformation have been inadequate (Steiner et al. 2015). Several applications of wheat straw in biotechnological processes have been reported, and this has been achievable on an industrial scale by adopting solid-state fermentation (SFF) or submerged fermentation (SMF) since wheat straw contains basic nutrients required for microbial growth (Mussatto 2014).
Citric acid (2-hydroxy-1,2,3-propanetricarboxylic acid) is the most important commercial product, which is found in almost all plant and animal tissues. It exists widely in nature and is present as a kind of fruit acid in lemon, orange, pineapple, plum, peas, and peach and animal bones, muscles, and blood. It has many applications in the food, pharmaceutical, and cosmetic industries as an acidulant, flavor enhancer, preservative, antioxidant, emulsifier, and chelating agent (Książek 2023). In recent years, citric acid has been commercially produced by fungal fermentation mainly byAspergillus niger(Książek 2023). Poultry meat, which is believed to be a perishable product is highly susceptible to spoilage in the form of discoloration, off odours/taste, and altered viscosity during storage at ambient conditions. Foodborne illnesses resulting from poultry meat contamination have also become a major source of global concern. Salmonella and Campylobacter cause more foodborne illnesses in poultry than any other bacteria (Hafez and El-Adawy 2019). It was estimated that one in every 25 packages of chicken at the grocery store is contaminated with Salmonella (CDC 2022). Verotoxin-producing Escherichia coli O157:H7 (VTEC), Listeria, and Yarsinia have become prominent in some areas as additional foodborne pathogens. A number of other toxigenic pathogens such as Clostridium perfringes, Bacillus cereus and Staphylococcus aureus can also enter the food chain via contaminated poultry products. Several regulations have been passed by the European Parliament Council Regulation (EPCR) on the control of several foodborne zoonotic agents, which covers the adoption of certain regulations aimed at reducing the prevalence of specified zoonosis in food animals at the level of primary production. These infections are distributed worldwide and result in severe economic losses when no effort is made towards their control. It is therefore paramount to employ proper and adequate methods in preservation to prevent such changes and hence prolong its storage time (Hafez and El-Adawy 2019).
The application of organic acids in food preservation has since been considered. Researchers have investigated the efficacy of applying organic acids on meat surfaces during storage (Da Costa et al. 2019; Barcenilla et al. 2022). Various studies have also been carried out to evaluate the antimicrobial effects of certain acids-producing bacteria on the surfaces of meat products (Casas et al. 2021). Microbial proliferation and chemical spoilage are the two major causes of reduced shelf-life in fresh poultry meat during refrigeration storage, therefore the employment of adequate preservative agents in the treatment of meat surfaces could go a long way in inhibiting microbial growth. Owing to their ability to alter the proton motive force (PMF) generated on the cell surfaces of microorganism’s organic acids have the potential to be highly effective in meat preservation if applied optimally in meat treatments (Van Ba et al. 2018). Hence citric acid produced from lignocellulosic waste using A. niger in submerged fermentation can be used as a preservative agent, which will not only help to reduce environmental wastes but will also preserve meat from post-slaughter spoilage.
Therefore, this study aims to assess the ability of citric acid produced by wheat straw fermentation with A. niger, in extending the shelf life of fresh poultry meat.
MATERIALS AND METHODS
Materials and reagents
The wheat straw was collected from farms within Ondo and Osun state in southwestern Nigeria. The collected samples were cut into pieces, milled (Jinsong, China), and sieved to obtain 40-60 mesh fractions. The samples were then homogenized and stored in plastic bags for further use. Poultry meat samples were obtained from freshly slaughtered chickens at a local poultry farm in Akure, Ondo state, Nigeria. NaOH, HCl, distilled water, plate count agar, violet, red bile glucose agar (VRBGA), glucose, urea. All reagents used were of Sigma brand, Darmstard, Germany.
Microorganism
Aspergillus niger (OQ607797) and Trichoderma viride (OQ686701) used in this study were cultured in the Department of Microbiology, Federal University of Technology, Akure, Nigeria. The microorganisms were maintained on PDA (Potato-Dextrose agar).
Sample preparation
Poultry meat samples were obtained from freshly slaughtered broiler chickens (pH=6), the meat samples were aseptically deboned, defatted, and cut into strips, using sterilized utensils. Prepared meat strips were then packed into sterile polyethylene bags, sealed, and rapidly transferred to the laboratory in ice packs for immediate treatment.
Inoculum preparation
Aspergillus niger was grown in Erlenmeyer flasks with 100 mL of liquid media containing; glucose, 20 g L-1; (NH4)2SO4, 2 g L-1; ZnSO4·7H2O, 0.05 g L-1; FeSO4, 0.018 g L-1; KH2PO4, 0.3 g L-1; and MgSO4, 0.3 g L-1. The flasks were incubated on an incubator shaker (MRC Laboratory Instruments, Israel) continuously at 160 rpm and 35 °C for 18 hours before use (Kou et al. 2013).
Dilute acid pretreatment
Dilute acid pretreatment of wheat straw was carried out using a modified method by Mood et al. (2013). Previously milled samples straw was deacetylated using a dilute NaOH (0.4% w/v) at 80 °C for 2 h, after which solids were washed with water and then dilute H2SO4 solution was added to achieve a 0.8% (w/w) acid concentration for dilute acid pretreatment. The slurry was vigorously stirred for 2 h at room temperature, dewatered to approximately 40% solids, and then incubated in a horizontal pretreatment reactor at 140 °C with a residence time of 10 min. After pretreatment, the material was then separated into the slurry stream with high solid content and a volatile flash vent stream. Pretreated deacetylated dry slurry was then neutralized using a 50% NaOH solution.
Enzyme extraction and assay
The fungi specie Trichoderma viride, was used as a source of cellulases. For cellulases production, 150 mL liquid medium containing: (NH4)2SO4 (1.4 g L-1); Urea (0.3 g L-1); KH2PO4 (2.0 g L-1); MgSO4.7H2O (0.3 g L-1); CaCl2 (0.3 g); Tween 80 (0.2%); wheat straw powder (20 g); cellulose powder (8 g); and 1 mL trace element solution (Alrumman 2016), was added in 250 mL conical flask. Each flask was inoculated with 2x108 Trichoderma viride spore suspension. Enzyme production was carried out at 30 °C and pH=7 in an incubator shaker with a speed of 130 rpm for 96 h. The culture medium was then harvested by centrifugation at 8,000 rpm for 10 min at 4 °C (MRC laboratory instruments, Israel). The supernatant was then used as the source of cellulose and enzyme activity was then determined (Zhao et al. 2012).
Enzymatic hydrolysis
Enzymatic hydrolysis of pretreated slurry was carried out using the method of Holtzapple (2003). 100 g of slurry was diluted by the addition of process water to 10% total solids. The diluted slurry was mixed with an appropriate amount of the clarified enzyme (30 FPU g-1 of pretreated substrate slurry) in a sterile fermenter containing 0.05 M acetate buffer (pH=5). Hydrolysis of the substrates was carried out at 50 °C for 72 h and agitation speed of 100 rpm (Zhang et al. 2012).
Submerged fermentation
The fermentation medium used for this study was composed of carbon source (wheat straw hydrolysate) supplemented with KH2PO4 (0.5 g L-1); ZnSO4·7H2O (0.05 g L-1); MgSO4.7H2O (0.3 g L-1); CaCO3 (30 g L-1); NH4NO3 (2 g L-1) (Huang et al. 2006). The agitation speed was maintained at 180 rpm (Ngouénam et al. 2021).
Fermentation procedure
Wheat straw hydrolysate from above was supplemented with the required nutrients. The pH was adjusted using HCl (1 N) and NaOH (2 M, pH=5). Thereafter, 10% inoculum size was aseptically added, and the medium was covered. The medium was then incubated at 35 oC for 7 days (Azaizeh et al. 2020). The fermentation medium was then centrifuged, and the supernatant was analyzed for citric acid.
Determination of reducing sugar and citric acid content
The reducing sugar content was quantified using the DNS assay method, while citric acid levels were determined via High-Performance Liquid Chromatography (HPLC) (Shimadzu, Japan) with a C18 column and IR detector. Sulfuric acid at 0.7 mL min-1 was used as the mobile phase. The detection was carried out at 210 nm (Wang et al. 2017).
Acid-soaking of fresh poultry meat
Citric acid was diluted using distilled water to achieve desired concentrations (1, 2 and 3%). The solutions were then used to soak the previously prepared meat samples, meat samples were also soaked in distilled water under similar conditions and used as a control. Treated samples were packed in HDPE film and stored at 4 oC for 14 days (Kang et al. 2003).
Variation of meat soaking parameters
Meat soaking parameters were varied according to the method of Xiaowei et al. (2015). Different acid soaking times (5, 10, 15, 20, and 25 min) and acid-soaking temperatures (10, 20, 30, 40, and 50 °C) were evaluated for their effect on the pH of treated meat before and during storage.
Meat acid activity determination
Treated meat samples (10 g) were homogenized in 90 mL of distilled water. Subsequently, the mixture underwent centrifuging, and the resulting supernatant was collected for titration using a standard NaOH solution (0.001 mol L-1) (Hatcher et al. 2004).
Microbiological quality of treated meat samples
The microbiological quality of both treated and untreated poultry meat samples was assessed using culture-dependent methods involving plate counts. Total viable counts (TVC) and Coliform counts were conducted daily over a span of 14 days, following the protocols outlined by Yang et al. (2016). Specifically, 10 g of meat sample was aseptically plated onto appropriate agar medium and incubated at 37 °C. Plate count agar and violet, red bile glucose agar (VRBGA) were utilized for TVC and TCC determination respectively.
Determination of total volatile basic nitrogen (TVB-N) and thiobarbituric acid reactive substances (TBARS)
TVB-N was determined according to the procedures described by FAO (1986). Meat samples were distilled into a 2% boric acid solution and titrated with 0.1 N H2SO4 (titer). TVB-N (mg N 100 g-1 flesh) was then calculated using the equation 1:
TBARS was determined according to the method of Schmedes and Hølmer (1989).
Meat samples were mixed with 25 mL of trichloroacetic acid (20% w/v) and filtered. The filtrate was then incubated with aqueous thiobarbituric acid at boiling temperature for 30 min, after which, the absorbance was measured at 532 nm using a UV- spectrophotometer. TBARS estimates were expressed as mg malondialdehyde (MDA) kg-1 of broiler fillet sample.
Sensory evaluation of treated poultry meat samples
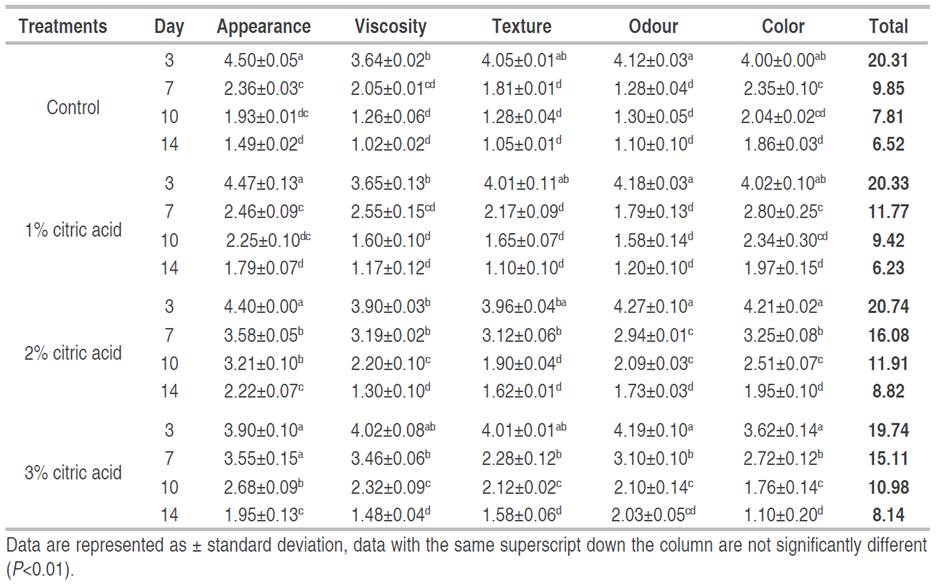
Sensory evaluation of the treated meat samples was carried out at the Department of Microbiology, Federal University of Technology, Akure Ondo State, Nigeria. The sensory attributes evaluated include the appearance, viscosity, texture, color, and odour of the meat samples. The evaluation was carried out on a total of four treatments, by a 12-member semi-trained panel, using a 5-point hedonic scale (Chen et al. 2019). The scale utilized ranged from 1 to 5, with details provided in Table 1 below. The 12-member panelists were carefully drawn from members of the university community comprising of students and staff. The panelists were made up of seven females and five males all between the ages of 20 and 50. Before the sensory evaluation, the panelist was tasked with evaluating each sample independently, without comparison to others. A minimum total score of 17 was set as the threshold for considering the sample fresh, while a score of 12 was deemed the lowest acceptable threshold.
Statistical analyses
All analyses were performed in triplicates after which the results were presented by means with standard deviation. Data were displayed as mean values attached to the standard deviation (One-way ANOVA). Duncan’s new multiple range test (P<0.05) was employed for the determination of significant differences between means, using the SPSS 20 statistics software (2020, IBM, Chicago, Ill., U.S.A.).
RESULTS AND DISCUSSION
Properties and yield of all fermentation parameters involved in citric acid production
Table 2 shows the cellulase activity of the crude enzyme obtained from T. viride, FPase was 6.25 U mL-1, endoglucanase activity was 8.5 U mL-1, and β-glucosidase activity was 5.0 U mL-1. The On-site cellulose produced by T. viride was found to effectively hydrolyze available cellulose fractions in wheat straw. This result agreed with the findings of Zhao et al. (2012).
Figure 1 shows the retention time and peak representing the products generated (citric and oxalic acids) from A. niger fermentation. Citric acid was identified at 11.921 min with a peak area of 1120.827, while oxalic acid, which is a by-product of citric acid fermentation was also identified. The citric acid yield obtained as shown in Table 3 was 14.15 g L-1 which cumulated to 26.18% (w/w). These results contrast with the findings of Ramesh and Kalaiselvam (2009), who reported citric acid from A. niger with a value of 50.0 g L-1 and a percent yield of 70.4%. Auta et al. (2014) reported a value of 1.15 g L-1 with a higher yield of 22.5% from Parkia biglobosa. The higher yield of citric acid in their study could be attributed to the use of better-adapted strains of A. niger which facilitated higher sugar-to-acid conversion rates. Ozen and Ozilgen (1992) also noted that low enzymatic activity as recorded in this study, is capable of limiting the saccharification process which in turn could affect the overall yield of citric acid. The high pKA of citric acid, its potential as a pH regulator, and its antibacterial activity make it a good preservative agent (Thangavelu and Murugaiyan 2011).
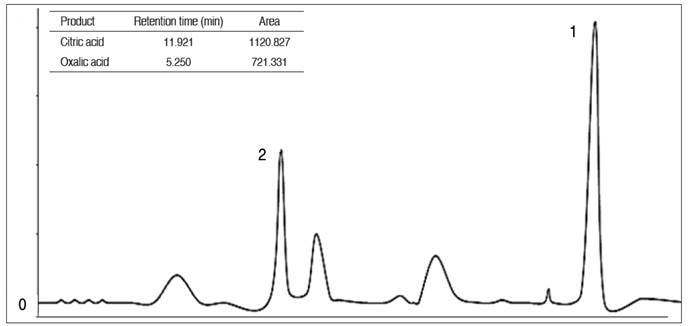
Figure 1 HPLC chromatogram for Aspergillus niger production of citric acids from wheat straw. (1 = Citric acid and 2 = Oxalic acid).
Over the years, low-cost agro-residues have been effectively utilized in the production of organic acids by fungi through submerged fermentation (Gao et al. 2013). Wheat straw is an interesting biomass with an abundance of cellulose and hemicellulose which can be converted to citric acid with different organisms through co-fermentation strategies (Ogidi et al. 2020).
Effect of acid-soaking time and temperature
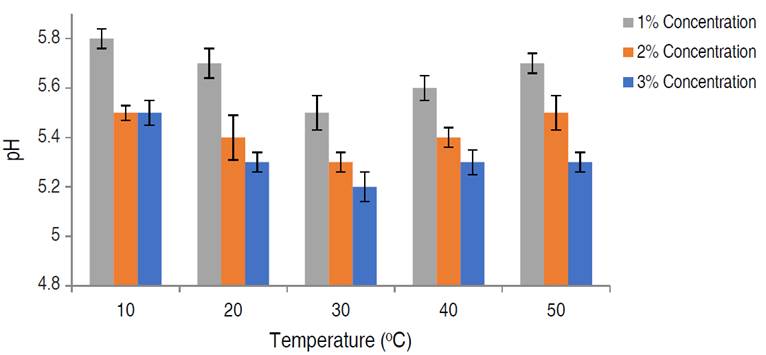
The effect of soaking time and temperature on the initial pH of the meat samples was estimated using varied acid treatment concentrations (1, 2, and 3%) (Figures 2 and 3). The acid-soaking time was positively related to the initial meat pH. It was observed that an increase in the acid-soaking time resulted in a corresponding decrease in the initial pH of the meat samples. The lowest pH of 5.2±0.20, 5.4±0.20, and 5.6±0.10 was observed after 20 min of soaking with 3, 2, and 1% citric acid solution, respectively. This was regarded as the optimum soaking time. The acid-soaking temperature was also positively related to the initial pH of the meat samples up to 30 °C. Further increases in temperature beyond 30 °C led to a rise in the meat pH and hence reduced acidity, because the organic acids used were highly volatile at temperatures above 30 °C, hence drastically reducing their effectiveness (Ren et al. 2012). The lowest pH of 5.2±0.10, 5.3±0.20, and 5.5±0.10 were observed at 30 °C soaking temperature with 3, 2 and 1% citric acid solution, respectively. This was also regarded as optimum. Pre-storage conditions of meat have been identified as one of the major factors that influence the keeping quality of the meat samples. Food processors have resulted in salting, drying, etc., in a bid to achieve optimal pre-storage conditions in meat (Kang et al. 2003). Varying the acid-soaking time and temperature of the meat treatment, had a significant effect on the initial pH of the meat samples (P<0.05). The observed decrease in pH at optimal soaking parameters of 25 min and 30 °C, respectively, enabled the establishment of ideal pre-storage conditions in the meat samples. Reduction in pH (which is a major factor influencing microbial growth) to unfavorable levels have been found to directly improve the keeping quality of most food substances (Sánchez-Clemente et al. 2018). It was noted that meat treatment with 3% citric acid concentration had the greatest effect on the pH reduction of meat prior to storage.
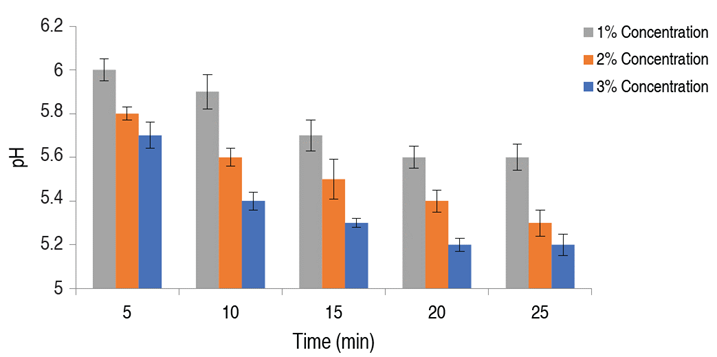
Figure 2 Effect of citric acid soaking time on the initial pH of poultry meat. Significance (P<0.01).
Microbiological quality of meat
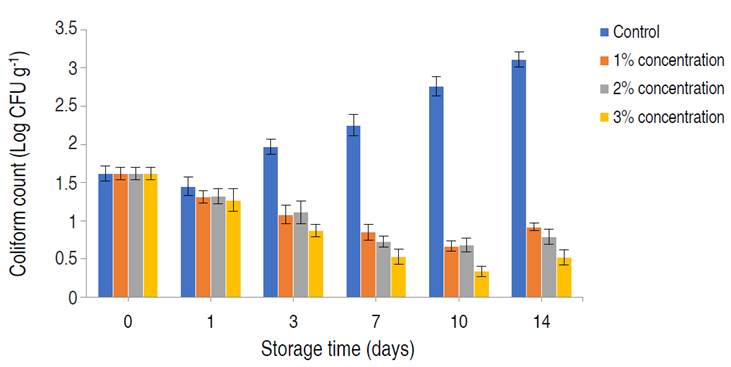
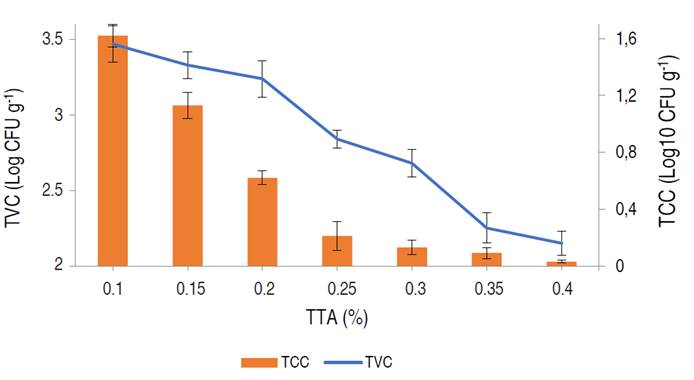
The shelf-life and safety of the preserved poultry meat were evaluated by estimating the TVC and TCC of the meat treated with different acid concentrations (1, 2, and 3%) at 25 min acid soaking time and 30 °C acid soaking temperature for 14 days (Figure 4 and 5). Reduction in TVC was directly proportional to the acid treatment concentration used. The highest counts were observed in the control samples, in which the TVC was observed to increase during storage. The lowest TVC of 2.55±0.25 Log10 CFU g-1 was observed on day 8 of storage, using a 3% concentration. However, TVC in all samples was observed to increase after day 8 of storage. There were significant differences in the TVC of the meat samples treated with different acid concentrations (P<0.05). TCC was also lowest when a 3% citric acid concentration was used. The highest coliform counts were recorded in the control samples, which showed a steady increase in coliform bacteria during storage with the highest TCC of 3.11±0.05 Log10 CFU g-1 on day 14. Citric acid treatment resulted in considerable reduction in TCC during storage, lowest TCC of 0.34±0.04 Log10 CFU g-1 was observed on day 7 using 3% acid concentration. This was similar to the work of Tian et al. (2022), who employed lactic acid in the treatment of beef. An increase in TCC in all samples was observed after day 10. There were significant differences in the TCC of the meat samples during storage with different treatments (P<0.05). TVC and TCC are often regarded as direct quality indicators in food samples and have been proven to have a positive correlation to the food spoilage process and food safety respectively (Zhang et al. 2021). The initial bacterial count in meat samples were within the acceptable range (6.0 Log10 CFU g-1), indicative of proper meat handling/hygiene (Santos et al. 2018). TVC and TCC values of 6.0 and 2.0 Log10 CFU g-1 are regarded as the threshold for fresh meat acceptability by the International Commission on Microbiological Specifications for Foods (ICMSF 2022), hence, rendering the control samples completely unacceptable beyond day three. The observed reduction in the rise in TVC and TCC within the acid-treated samples as storage progressed was significantly influenced by acid treatment concentration. This reduction in pH can severely affect the growth and survival of non-acidophilic bacteria, which are the group predominantly responsible for meat spoilage and infection (Tian et al. 2022). This effect can be attributed to a disruption in pH homeostasis, which is highly critical in microbial metabolism; due to its role in maintaining the proper function of biological macromolecules as well as maintaining the kinetic and thermodynamic force of chemical reactions involving protons as metabolites (Sánchez-Clemente et al. 2018). Although citric acid exhibited good bacteriostatic and bactericidal effects on treated meat samples, 3% citric acid treatment was; however, most effective. Odu et al. (2020), suggested that certain organic acids such as lactic and citric had high bacteriostatic effect due to their pKA value (3.1 for citric acid), displaying less dissociation than others, hence making them more lethal to bacteria.
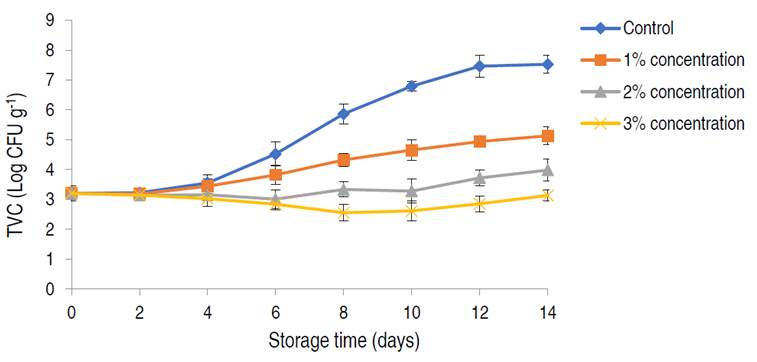
Figure 4 The total viable count of poultry meat treated with different concentrations of citric acid and stored for 14 days. Significance (P<0.05).
Changes in pH and acidity activity of samples during storage
Figure 6 shows the changes in pH of poultry meat stored for 14 days using different concentrations of citric acid at 25 min soaking time and 30 °C soaking temperature. The enduring effect of the acid treatment process on the meat samples during storage was further confirmed by the observed changes in pH in both the treated and untreated meat samples. A general decrease in the pH of treated meat samples was observed up until day 8, this was contrary to the findings of Han et al. (2020) who recorded fluctuations in the pH of broiler meat spread with lactic acid. The lowest pH of 5.0±0.10 was observed using a 3% citric acid concentration on day 12. The pH of the control sample on the other hand was observed to increase as storage progressed beyond day 12 and this can be attributed to the observed increase in microbial growth at this stage of storage (Gao et al. 2013). There were significant differences in the pH of the meat samples treated with varying concentrations of acid as storage progressed (P<0.05). Higher acid treatment concentrations led to lower meat pH during storage. The pH of meat plays a vital role in its quality and shelf-life during storage, and any deviations from the acceptable range can result in adverse effects on the color/appearance and water-holding capacity of fresh meat (Han et al. 2020). pH fluctuations of broiler meat during storage have been found to greatly affect the production of sulfur-containing and carbonyl volatiles. Extreme alkaline and acidic conditions in meat during storage greatly increase the production of these volatiles. At these extreme conditions, darkening tends to occur resulting in meat discoloration and loss of flavor (Haščík et al. 2013). The optimal pH for the preservation of poultry meat was between 4.5 and 5.5, it was however evident that meat treatment with 2 to 3% citric acid solution led to delayed glycolysis and effectively prevented rapid meat acidification at the early stages of storage; these treatments were also observed to efficiently prevent undesirable pH fluctuations in the treated meat samples at the mid and late stages of storage (Holman et al. 2016).
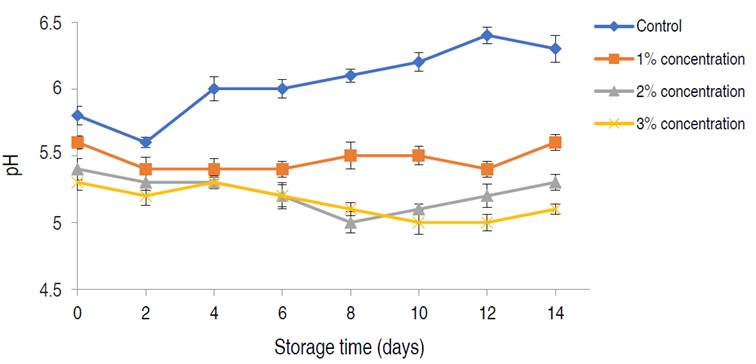
Figure 6 pH changes of poultry meat preserved with different concentrations of citric acid for 14 days. Significance (P<0.02).
The correlation between the meat acid activity, TVC, and TCC is shown in Figure 7. The higher the meat acid activity, the lower the TVC and TCC, the lowest TVC of 1.95±0.08 Log10 CFU g-1 was observed at an acid activity of 0.4%, while the lowest TCC of 0.03±0.01 Log10 CFU g-1 was observed at same acid activity. Reduction in TVC and TCC during storage showed significant differences in comparison with the acid activity of the treated meat samples (P<0.05). Since the acid activity of the meat samples was a cumulative effect of all treatment parameters, it was conceivable that the observed increase in antimicrobial activity in the form of TVC and TCC reduction was a direct effect of the use of optimal treatment conditions (in relation to treatment time and temperature) (Zhang et al. 2021).
Anti-oxidative effect of citric acid treatment on poultry meat samples
Table 4 shows the effect of different concentrations of citric acid treatments on the formation of Total Volatile Basic Nitrogen (TVB-N) and Thiobarbituric Acid Reactive Substances (TBARS) in poultry meat samples during storage. TVB-N and TBARS content in chicken as an important reference index has been used to evaluate its freshness (Castro et al. 2006). TVB-N compounds in chicken contain ammonia, trimethylamine (TMA), and dimethhylamine (DMA), and the level of TVB-N compounds increases with spoilage by either bacteria or enzymatic degradation. TBARS is an index of lipid oxidation, measuring malondialdehyde (MDA) content, which is one of the degradation products of lipid hydroperoxides formed through the oxidation of unsaturated fatty acids (Gatellier et al. 2009).
Table 4 Changes in TVB-N and TBARS content of poultry meat treated with different concentrations of citric acid and stored for 14 days.
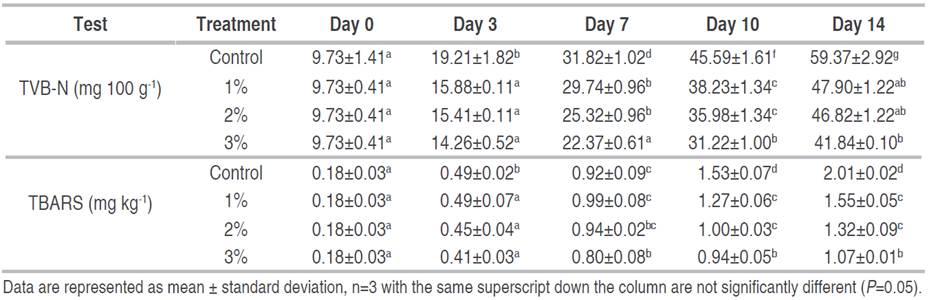
It was clear that there were no significant differences in both TVB-N and TBARS between treatments (P<0.05) on day zero. A significant increase in storage time (P<0.05) in both parameters was observed in all treatment groups. This agreed with Alasnier et al. (2000), Rukchon et al. (2014) and Rahman et al. (2012) who recorded similar differences as storage progressed. Significant differences (P<0.05) were again observed between the control and citric acid treatment groups. The control groups showed the overall highest values with a maximum of 59.37±2.92 mg 100 g-1 TVB-N and 2.01±0.02 mg kg-1 TBARS on day 14. An overall reduction in the formation of both TVB-N and TBARS was observed in the treated meat samples during storage as compared to the control (untreated) samples. 3% citric acid treatment had the highest effect in TVB-N and TBARS reduction with 41.84±0.10 mg 100 g-1 and 0.97±0.01 mg kg-1 of meat samples on day 14, respectively. A clear relationship was observed between the microbiological quality of the treated meat samples and the levels of TVB-N and TBARS formation, this was again in agreement with Smaoui et al. (2012) who noted that reductions in TVC resulted in similar reductions in the formation of TVB-N. Since TVB-N is a function of protein breakdown, the observed increase may be attributed to the formation of ammonia, which could be a result of residual microbial activity in the meat samples during storage (Khalafalla et al. 2016). TVB-N values of all treated groups were above the limit of 40 mg 100 g-1 recommended by FAO (1986) at day 14 of storage. Likewise, all treatment groups had TBARS values above the permissible limit of 0.9 mg MDA kg-1 recommended by the United States Department of Agriculture (FAO 1986) at day 14 of storage.
Effect of acid treatment on sensory parameters
The sensory parameters of untreated and treated poultry meat with different concentrations of citric acid (1, 2, and 3%) at 25 min soaking time and 30 oC soaking temperature for 14 days are shown in Table 5. There were significant differences in sensory parameters of meat samples treated with varying acid concentrations (P<0.05). Citric acid treatment had positive effects on the sensory parameters of poultry meat when compared with the control (untreated) samples. There were no significant differences between the treatments and control samples at day 3 (P<0.05); all samples-maintained freshness, and this was an indication of the ability of citric acid treatments to extend poultry meat shelf-life, without adversely affecting its sensory quality (Maaya and Al-Abdullah 2016). On the other hand, significant differences ( P≤0.01) were observed in the sensory parameters of treated and untreated meat samples, as well as between different treatment concentrations from day 7. Only samples treated with 2% acid concentration and above were able to maintain freshness at day 7, (Chen et al. 2019). On day 14, all samples had sensory scores below the acceptable limit of 12, making them unacceptable. 2% citric acid-treated groups were observed to be the most effective in maintaining desirable sensory parameters (16.08 as of day 7). The maintenance of sensory parameters observed in the acid-treated groups were probable due to one or more carboxylic acid or acid phenolic groups present in the treatment acids such as amides, esters, and peptides (Carpes et al. 2009). These carboxylic groups play a functional role in lipids and protein metabolism and acid-base balance, thereby positively influencing the sensory parameters of poultry meat (Haščík et al. 2013). These findings were in accordance with Bobko et al. (2012), who found a significant positive influence of different plant supplements containing organic acids on the sensory quality of poultry meat.
CONCLUSION
Citric acid produced from the fermentation of wheat straw with A. niger significantly inhibited the proliferation of spoilage organisms as well as the rate of protein and lipid oxidation in treated meat samples. It was found that untreated poultry meat had a maximum shelf-life of 4 days at 4 °C, while poultry meat treated with 2% citric acid and above was preserved for up to 10 days. The presence of one or more carboxylic acid or acid phenolic groups present in citric acid such as amides, esters, and peptides make it an efficient meat preservative agent. This study gives insight into the further industrial application of lignocellulosic biomass with an emphasis on food preservation. However, less expensive conversion and purification techniques should be explored to make the whole process more feasible.