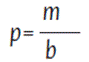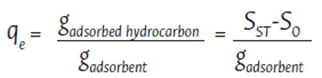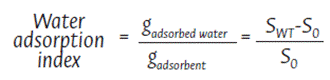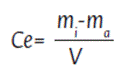Introduction
The industrial revolution marked the onset of hydrocarbon compound spills which, to this day, still degrade the soil, flora, and fauna (Zhang et al., 2019). Adsorbent materials capable of capturing and removing compounds threatening the stability of the environment may offer a solution to this issue, as they are seen as a viable alternative to mitigate the environmental consequences of these spills (Patalano et al., 2019).
The use of adsorbent materials is a promising approach to remove, control, and reduce hydrophobic and oleophilic substances with notable retention times of pollutant loads. However, to effectively address the release of hydrocarbon compounds from the industrial sector, adsorbent materials must be cost-effective. Various adsorbent materials, both synthetic and inorganic, have been proposed as alternatives. Natural fibers from agro-industrial wastes, such as wood chips, tree barks, coconut shells, rice straws, and cane bagasse, have gained attention due to their high adsorption capacity and biodegradability (Bhardwaj and Bhaskarwar, 2018; Díaz-Díaz et al., 2018). While synthetic adsorbents offer excellent removal performance, they tend to be expensive and can have environmental impacts.
In Ecuador, where the economy is mainly driven by agriculture, significant amounts of waste are generated daily from the processing of bananas, cocoa, and rice (including shells, leaves, rachis, and stems), making them promising sources of low-cost and effective adsorbent materials for hydrocarbon compounds (Gupta et al., 2022). Approximately 20 % of the agro-industrial wastes in the country are generated in El Oro province (Martínez-Altamirano and Flores, 2014); recycling and reusing these wastes as adsorbent media for hydrocarbon compounds aligns with the principles of the circular economy. However, to be considered a suitable adsorbent material, an agro-industrial waste must meet certain criteria established in international standard tests, such as ASTM F726-12 (Silva et al., 2021).
The aim of this study was to evaluate the adsorption capacity of agro-industrial wastes from banana leaves and rachis, cocoa husk, and rice husk for hydrocarbon compounds, as well as their potential use in pollution control processes within water bodies, utilizing the ASTM F726-12 standards.
Material and methods
A total of five different agro-industrial wastes, namely cocoa husks, rice husks, banana leaves, banana peels, and banana rachis, were collected and subjected to preliminary treatment. The wastes were initially chopped into 5 cm pieces and dried for 30 days at room temperature. The dried materials were subsequently milled and sorted into two different particle sizes, namely 850 µm and 1400 µm, as described by Díaz-Díaz et al. (2018).
A chemical treatment was carried out on 50 g of each dried waste material, which were soaked in 500 mL of 0.1 M NaOH for 30 minutes. The soaked plant matrix was then removed from the beaker and placed into a laboratory oven at 100 °C for 2 hours to remove the cellulose content present in the vegetable tissues and create space for hydrocarbons to be adsorbed. To characterize the prepared adsorbent materials, the apparent density of each sample was measured by filling a test tube previously weighed with a sample of each material up to 100 mL and weighing the filled test tube. The apparent density of each sample from each material at both particle sizes was determined using Equation 1:
Where:
p = Apparent density of the adsorbent material
m = Mass of the adsorbent material
v = Volume within the test tube
The buoyancy tests were performed following the ASTM F726-12 standard. Specifically, 3 g of each adsorbent material at the two different particle sizes were introduced into a 1000 mL beaker containing 500 mL of distilled water and allowed to settle for 24 hours. The sinking fractions were observed and registered.
To assess the maximum amount of hydrocarbon adsorbed in 30 minutes and 24 hours, short-term and long-term hydrocarbon adsorption tests were conducted in accordance with the ASTM F726-12 standard (ASTM, 2012). Three types of hydrocarbon compounds were used for testing purposes: commercial motor oil (composed of 78 % base oil, 10 % viscosity improvement additive, 3 % detergent, 5 % dispersant, 1 % wear protection, and 3 % of other components), diesel, and gasoline. A 1000 mL solution was prepared containing 20 % of each hydrocarbon compound, filling the remaining 80 % with water. For the short-term and long-term hydrocarbon adsorption tests, 3 g of each adsorbent material was added to a beaker containing 300 mL of each hydrocarbon solution. After the specified time for each test (30 minutes and 24 hours, respectively), to evaluate the performance of the adsorbent material in static conditions, the soaked adsorbent material was removed with the help of a mesh size 4 (US mesh size), let dripped for 20 minutes at room temperature, and weighed in an analytical balance (AES 200, Kern & Sohn GmbH, Balingen, Germany). The adsorption capacity at equilibrium (qe) for hydrocarbon compounds was determined using Equation 2:
Where:
qe = Adsorption capacity at equilibrium of the material tested
S0 = Initial mass of the dry adsorbent
SST = Mass of the adsorbent after the adsorption test
To evaluate the performance of the adsorbent material in dynamic conditions, a procedure analogous to the short-term and long-term hydrocarbon adsorption tests was carried out under constant agitation using a magnetic stirrer (PCE Instruments UK Ltd., Southampton Hampshire, United Kingdom) operating at 100 rpm. The water adsorption index of each adsorbent was calculated using Equation 3:
Where:
SWT = Mass of the adsorbent material after the dynamic test
S0 = Initial mass of the dry adsorbent material
The results of the tests carried out were arranged in a Latin square design with 2 factors and 3 variables, whereas the adsorption capacity was set as the response variable. The analysis of variance (ANOVA) was applied with the Statgraphics Centurion XVI statistical package (Statgraphics Technologies, Inc., Virginia, United States). On the other hand, to assess the results of the short-term and long-term adsorption tests under static conditions at the 2 particle sizes defined, 4 Latin squares were established. To determine the adsorption rate for hydrocarbon compounds in each adsorbent material, the ratio between the contact time and the adsorption capacity was evaluated. The same procedure used in the adsorption capacity tests was applied. The adsorption capacity was recorded after 1 minute and then every 5 minutes until equilibrium, determined after 30 minutes (Daud and Hussin, 2023).
To plot the adsorption isotherms, the adsorption capacity was defined with a variation: the adsorbent amounts were modified while the amount of adsorbate remained. The amounts of adsorbent used were 2, 3, 4, 5, and 6 g. The adsorption constants for the Langmuir and Freundlich models were obtained using the equations described above. The adsorption capacity in the adsorbate over the adsorbent (qe) was calculated using equation 2 (g/g) and the equilibrium concentration of the adsorbate in the initial mass of the hydrocarbon tested (Ce), expressed in (g/L), was calculated after Equation 4:
Where:
Ce = Equilibrium concentration
m i = Adsorbent mass and adsorbate mass
mα = Hydrocarbon mass adsorbed by the adsorbent V = Volume of the solution m i -mα
To determine the maximum adsorption capacity (qmax) and the Langmuir isotherm constant (KL), the relationship between the equilibrium concentration Ce and the ratio of Ce/qe was plotted. The slope of the resulting line represents 1/qmax, while the intercept represents 1/(KL*qmax). The determination coefficient R2 was obtained from the plot to assess the goodness of fit of the Langmuir isotherm model. For the Freundlich model, a plot was constructed with the logarithm of the equilibrium concentration (Log Ce) on the x-axis and the logarithm of the adsorption capacity (Log qe) on the y-axis. The slope of the line in this plot represents 1/n, where n is a constant related to the adsorption intensity, while the ordinate represents Log KF, which is a constant related to the adsorption capacity (Abin-Bazaine et al., 2022). All experiments were conducted with a systematic approach, employing 2 replicates for each treatment.
Results
Table 1 provides a codification scheme for identifying each adsorbent material based on its particle size. As for the buoyancy test, all samples demonstrated buoyancy in both pure water and water/hydrocarbon solutions, except for the cocoa shell adsorbent in both particle sizes, as indicated in Table 1. The methodology employed drop filtration without heat drying to estimate the hydrocarbon adsorption capacity. This approach was chosen under specific criteria to maintain the integrity of the adsorbent material. However, after careful consideration of potential alteration of adsorption properties and practical constraints, it was decided to omit vacuum filtration after the experiments.
When in contact with the liquid surface, the cocoa shell adsorbent material precipitated after approximately 5 minutes, with around 5 % of its particles floating at the end of the test. Hence, it was deemed unsuitable for use as adsorbent material in aqueous media and was therefore excluded.
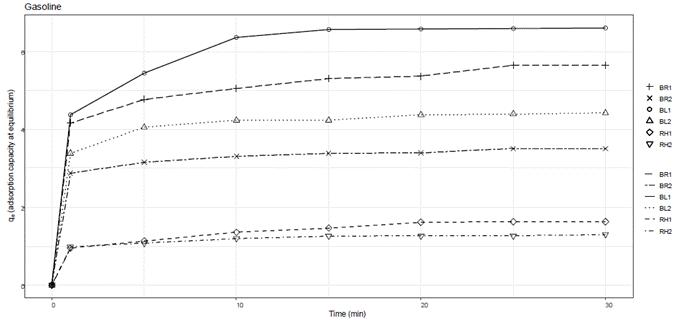
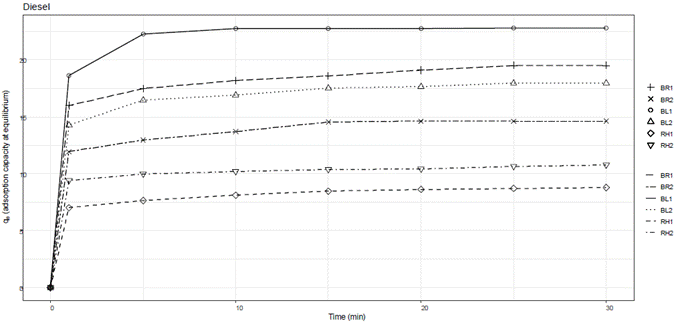
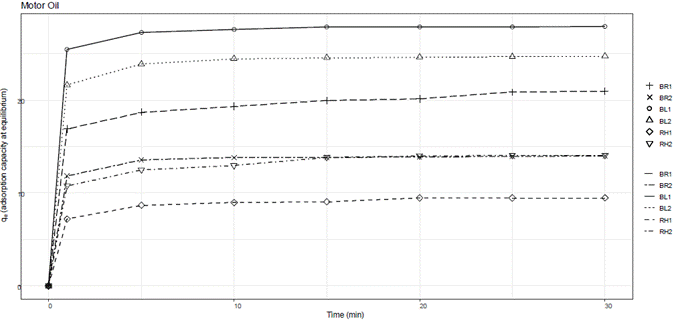
A graphical representation of qe versus time was generated to illustrate the dynamic behavior of adsorption over the specified time intervals for each hydrocarbon tested. Figure 1 depicts the dynamic behavior of adsorption over time for gasoline. Figure 2 depicts the dynamic behavior of adsorption over time for diesel. Figure 3 depicts the dynamic behavior of adsorption over time for motor oil.
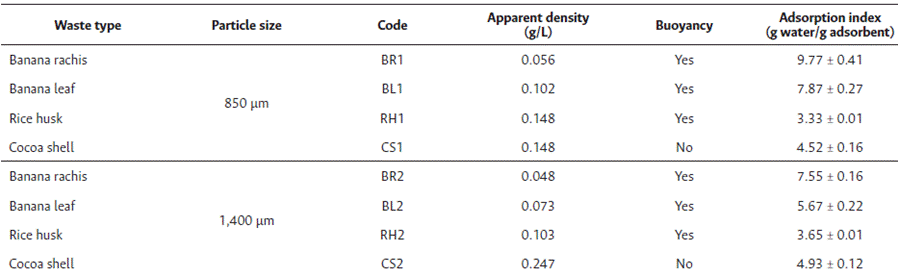
Banana leaves, in both particle sizes, presented a lower sinking percentage (between 5 and 7 %), standing as the best option for hydrocarbon adsorption in an aqueous medium. Regarding the analysis of bulk density, cocoa shell, in both particle sizes, presented a higher apparent density; this might be attributed to a deficiency in the flotation tests, while banana rachis presented a lower apparent density of around 0.048 - 0.056 g/mL, followed by banana leaves (0.073 and 0.102 g/mL), and rice husk (0.103 - 0.148 g/mL), resulting in lower apparent density values in adsorbents of larger particle size.
The adsorption test during the hydrophilic phase showed that approximately 90 % of the 1400 µm particle size adsorbent material obtained from banana leaves floated, while adsorbing 5.67 g water/g of adsorbent. This result falls within the expected range for bulk adsorbents. In terms of water adsorption index, the material obtained from banana rachis and banana leaves of 850 µm particle size exhibited the highest yields, with 9.77 g and 7.87 g water/g adsorbent, respectively. This was followed by banana rachis of 850 µm particle size with 7.55 g water/g adsorbent, and rice husk of 850 µm and 1400 µm particle size with 3.65 g and 3.33 g water/g adsorbent, respectively.
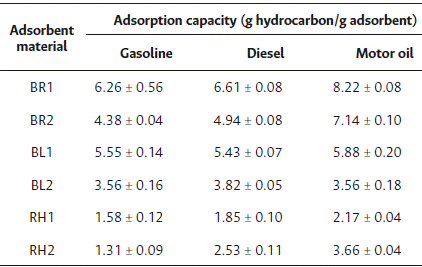
The oleophilic tests showed that banana leaf of 1400 µm particle size, a hydrophilic material, adsorbed all three types of hydrocarbon compounds tested; however, traces of these compounds were still visible in the aqueous media. In the short-term static tests, the adsorbent materials showed higher adsorption indices for motor oil and diesel; this behavior could be attributed to viscosity, as motor oil has a higher viscosity compared to the other two hydrocarbon compounds studied, whereas diesel has a higher viscosity compared to gasoline. Table 2 summarizes the results of the adsorption capacity in the short-term test. The adsorbent material from banana rachis in both particle sizes (850 µm and 1400 µm) demonstrated the highest adsorption capacity, reaching 8.22 and 7.14 g oil/g adsorbent, respectively. In contrast, the banana leaves material in both particle sizes presented an adsorption capacity of 5.88 and 3.56 g oil/g adsorbent, respectively, while rice husk of 850 µm had the lowest adsorption capacity (2.17 g oil/g adsorbent).
The adsorption capacity of the banana rachis adsorbent material with a particle size of 850 µm was 6.61 g diesel/g adsorbent. The other adsorbent materials tested had an adsorption capacity ranging from 1.85 to 5.43 g diesel/g adsorbent. The adsorbent material from rice husk with a particle size of 850 µm had the lowest adsorption capacity value (1.42 g diesel/g adsorbent).
The adsorbent materials from banana leaf and rachis of 850 µm particle size featured the highest adsorption capacity for gasoline, with values of 5.55 and 6.26 g gasoline/g adsorbent, respectively, as shown in Table 3. The adsorbent from banana rachis and banana leaf of 1400 µm particle size followed, with adsorption capacities of 4.38 and 3.56 g gasoline/g adsorbent, respectively. However, the adsorption capacity for rice husk of 850 and 1400 µm particle size was lower, at 1.58 and 1.31 g gasoline/g adsorbent, respectively. The adsorption capacity in the long-term tests was similar to that obtained in the short-term tests, except for the adsorbent material from banana rachis, which showed a higher adsorption capacity in the short-term test.
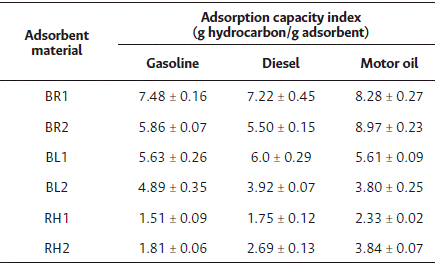
Similar to the short-term tests, motor oil was the hydrocarbon compound that was most effectively adsorbed by the majority of the adsorbent materials, with the exception of the adsorbent material from banana leaves.
The results of the categorical multifactorial ANOVA for the four Latin square designs indicated that the p-values were all less than 0.05. Therefore, the factors considered had a statistically significant effect on the adsorption capacity in both long and short-term tests. In other words, the variables played a crucial role in determining the adsorption capacity of each adsorbent material. The kinetic study revealed that, within the first minute of contact, the three adsorbent materials with buoyancy showed the highest amount of hydrocarbon adsorption. A slight increase in adsorption capacity was observed with extended contact time up to 30 minutes.
Gasoline was quickly adsorbed into the material surface via intermolecular forces, then transported into the material fibers through capillarity. A similar adsorption pattern was observed for diesel as well as for gasoline. Most of the adsorption occurred within the first minute, where a constant adsorption capacity was observed after 5 minutes for all adsorbent materials. Overall, the adsorbent materials tested demonstrated viable alternatives for adsorbing hydrocarbons with varied density and viscosity. As the amount of adsorbent material increases, its adsorption capacity decreases, with the adsorbent material from rice husk showing the lowest adsorption capacity and the highest amount of hydrocarbon remaining in the aqueous medium at equilibrium.
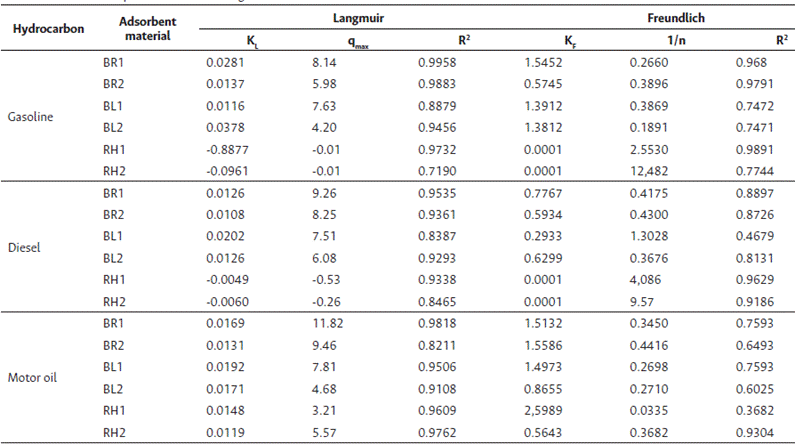
The adsorption capacity values were affected by the initial concentrations of hydrocarbon compounds. The adsorbent materials made from banana rachis and banana leaves showed higher adsorption capacity at equilibrium for all three hydrocarbon compounds tested, while the adsorbent material from rice husk showed differences between the three types of hydrocarbon compounds. The Langmuir and Freundlich models were used to analyze the experimental data. The Langmuir model showed a better fit for the experimental data, with a determination coefficient closer to 1, which indicates that the adsorption phenomenon occurred until the formation of a monolayer. The maximum adsorption capacity (qmax (g/g)) was found to be highest for the banana rachis of 850 µm particle size. The constant associated with the Langmuir isotherm related to the adsorption heat remained in a range between 0.0126 and 0.03678 L/g. However, for rice husk in gasoline and diesel, the Langmuir model was not acceptable as it resulted in negative values for the maximum adsorption capacity and the affinity constant, indicating a lack of physical significance.
The Freundlich model yielded lower determination coefficients compared to the Langmuir model. Nevertheless, the intensity of adsorption 1/n was in all cases, except for rice husk, less than 0.5, indicating that the adsorption was favorable. However, the experimental data for rice husk in both particle sizes did not conform to either the Langmuir or Freundlich models, suggesting that other models may better describe the adsorption phenomena in these materials. Table 4 presents the results of fit parameters of the Langmuir and Freundlich models.
Discussion
The findings of this research unveiled a trend indicating that larger particle sizes resulted in lower apparent density yields in the adsorbents; this pattern is consistent with previous reports on cane bagasse (Danglad et al., 2018). Regarding the dynamic tests, in comparison with Méndez-Tovar et al. (2012), the results of the present research showed higher water adsorption values, suggesting that the tested adsorbent materials possess hydrophilic surfaces, which may limit their effectiveness in hydrocarbon spill cleanup in water bodies, as they can adsorb both water and hydrocarbons. Consequently, their application may be more suitable for addressing spills on soil (Patalano et al., 2019). Traces of hydrocarbon compounds remained visible in the aqueous media during the oleophilic process suggesting that this type of adsorbent material may not be ideal for water body treatment. Sedimentation events could occur, causing hydrocarbon compounds to settle to the bottom due to the adsorbent material (Méndez-Tovar et al., 2012).
The highest adsorption capacity reached in this study is comparable to values reported for acetylated flax fibers (Mahmoud, 2020). The adsorption capacity of adsorbent material from banana rachis with a particle size of 850 µm was 6.61 g of diesel per gram of adsorbent, which was lower than sugarcane bagasse and coconut fiber (10.51 and 8.25 g of diesel per gram of adsorbent, respectively) (Abel, et al., 2020; Chau, 2021). The remaining adsorbent materials tested exhibited adsorption capacity values consistent with other reported values (Guilcamaigua-Anchatuña et al., 2019). The long-term tests showed adsorption patterns similar to the short-term tests, except for banana rachis, which exhibited higher adsorption capacity in the short term. This may be attributed to the affinity of banana rachis with water, as only a minimal portion of the adsorbent material sank to the bottom of the beaker after 24 hours (Méndez-Tovar et al., 2012).
The adsorbent materials tested exhibited varying buoyancy properties in pure water and hydrocarbon/water mixtures. Notably, materials derived from cocoa shells of 850 and 1,400 µm particle size did not show any buoyancy properties, whereas the hydrophilic phase in banana leaves of 1 400 µm particle size exhibited a buoyancy value higher than 90 %.
The choice of the adsorbent material plays a crucial role in its applicability for different spill scenarios. The apparent density of the materials was in the range of 0.048 to 0.148 g/L. Materials from cocoa shells had higher apparent density compared to other materials. This information is significant for understanding the handling and deployment of these adsorbent materials in real-world spill situations.
The trends observed in long-term tests were consistent with short-term tests, indicating the sustained effectiveness of these adsorbent materials over time. However, a portion of the adsorbent material from banana rachis subsided, adsorbing both water and hydrocarbon compounds, which may affect its practical use over extended periods.
Statistical analysis revealed significant differences (p<0.05) between the type of waste, the type of hydrocarbon, and their respective interactions. This underlines the importance of considering these factors when selecting adsorbent materials for specific spill scenarios.
The kinetic evaluation showed that each adsorbent material adsorbed the highest amount of hydrocarbon within the first minute of contact with the hydrocarbon-water mixture. This rapid adsorption rate is a valuable characteristic for efficient spill response.
Except for rice husk, each adsorbent material displayed a better fit with the Langmuir model. This suggests that the Langmuir isotherm can be a useful tool for predicting adsorption behavior for most of the tested materials. The Langmuir isotherm constant related to adsorption heat ranged between 0.0126 and 0.03678 L/g for the tested materials. This information can assist in understanding the energy and temperature-dependent aspects of adsorption processes.
It is recommended that future studies analyze hydrocarbon and water adsorption concentrations separately to elucidate the distinct contributions of each component to the overall adsorption process. This avenue of inquiry promises to deepen our understanding and potentially enhance the effectiveness of adsorption-based remediation techniques. Furthermore, incorporating a negative control in experimental designs is paramount for establishing a reliable baseline reference for the absence of the phenomenon under investigation. For instance, untreated samples or blank solutions serve as effective negative controls, which should undergo the same experimental conditions as the test samples to accurately reflect any effect.
To ensure the reliability of control data, it is advisable to replicate the negative control multiple times. This systematic approach aids in establishing a robust baseline for comparison, thereby enhancing the specificity of observed effects while minimizing potential confounding factors. Therefore, researchers are encouraged to incorporate rigorous experimental designs with appropriate negative controls in their studies to ensure the validity and reliability of their findings.
The results of the present research provided notable insights into the potential of tested waste-derived adsorbent materials for hydrocarbon spill mitigation. These materials show promising, particularly in short-term scenarios, but their effectiveness can be influenced by various factors, such as the material used and the specific spill characteristics. Further research and practical testing are warranted to fully assess their applicability in real-world spill situations and to optimize their performance. While our work focused on the adsorption capacity without specifically delineating between hydrocarbons and water, it is evident that further investigation into this matter would be beneficial for future research endeavors.
Conclusions
The tested materials showed high adsorption capacities for motor oil, with the highest capacity observed in the material prepared from banana rachis of 850 µm particle size. Additionally, the banana rachis waste material of the same particle size displayed the highest adsorption capacities for both diesel and gasoline. These findings suggest that these materials are promising for short-term mitigation of hydrocarbon spills.
The differentiation between the adsorption capacities for hydrocarbons and water could provide valuable insights into the efficiency and selectivity of the adsorbent material. Therefore, it is recommended that future studies consider analyzing hydrocarbon and water adsorption concentrations separately to elucidate the distinct contributions of each component to the overall adsorption process.