I. Introduction
Crude oil continues to be the main energy resource to respond to the growing global demand for energy, fuels and raw materials for industry. Figure 1 shows an annual oil demand growth between 1% and 1.8%, approximately [1]. On the other hand, renewable energy and its related research is still incipient [2]. In addition, the oil and petrochemical industry plays a key role in the economy and in the manufacture of various products [3].
There are several types of oil, which differ by their density measured in API grades (American Petroleum Institute) [4]. By means of API grades, crude oil can be classified as light (31.3-39°), medium (22.3-31.1°), heavy (10-22.3°) and extra-heavy (< 10°) [5].
Heavy oil is an abundant resource, corresponding to 70% the world's reserves [6]. However, it is difficult to extract, transport and refine, because its characteristics affect processing efficiency, increase operating costs, and generate greater environmental impact [6]. There are several difficulties for the processing of heavy crude oil, including: low API grade (< 20°), high viscosity (103 to 106 cP), and high concentrations of asphaltenes (10%p) and sulfur (1.3%p) [7]. Its physical and chemical characteristics are related to a higher concentration of asphaltenes and resins with respect to light crudes [8]. In spite of the difficulties it presents, this type of crude is necessary to satisfy the growing world demand for oil. In order to process this type of crude, it is necessary to reduce its viscosity through upgrading processes, which introduce important advantages, such as: facilitating transportation through pipelines, improving the performance of light products, increasing commercial value, and facilitating refining [9].
Upgrading consists of a set of techniques through which the hydrogenation of the molecules is achieved by the addition of hydrogen, which results in a lighter synthetic crude oil [8, 9]. Classic crude upgrading methods applied in surface operations include dilution and heating, which generally involve a very high investment in equipment and complex infrastructure, resulting in an increase in capital and operating costs [10].
There are also emerging technologies, which are based on principles such as: viscosity reduction, chemical changes, and friction mitigation between pipe and fluid. Some of them are relatively developed while others are in the research and implementation phase. Some methods involve catalytic cracking using ionic liquids, as well as the use of nanoparticles to improve the properties of the crude oil [11, 12,13]. Diaz et al.[10] and Castañeda et al. [11] present a complete review of emerging technologies for crude oil upgrading, classified into four categories: i) hydrogen addition, ii) carbon rejection, iii) extraction, and iv) ultrasound.
Ultrasound stands out among the emerging technologies, and it is used to generate the acoustic cavitation phenomenon [14], a technology that has potential benefits to facilitate the operation and handling of heavy crude oil [15]. Acoustic cavitation allows the release of high energy inside a liquid, which consequently induces catalytic chemical reactions and creates changes in the fluid properties, for example, viscosity reduction [15, 16, 17].
The purpose of this article is to present a literature review by various authors who provide empirical and experimental evidence for, and show the possibilities of, the reduction of crude oil viscosity by means of ultrasound.
Section II of the article presents the methodology of the study, while Section III describes the results and the general characteristics of ultrasound and acoustic cavitation, as well as the state of the art of research work applied to heavy oil, carried out by different authors over a period covering approximately 30 years.
II. Methodology
The document search was carried out using the following procedure: initially, the databases relevant to the subject were selected, such as Scopus, Science Direct, EBESCO, IEEE, Web of Science and OnePetro. Peer-reviewed publications from January 1970 to December 2019 were taken into account. The established Boolean algorithm for the search was the following:
(Ultrasound OR "ultrasonic waves" OR "acoustic cavitation" OR "ultrasound reactors" OR "Sonoreactor") AND (Petroleum OR "Heavy oil" OR "heavy crude oil") AND (Upgrading OR "viscosity reduction")
Figure 2 shows the record of documents related to the topic and their behavior over time and highlights how the theme has expanded in the last 10 years. This interest is justified by the fact that light oil reserves have started to become scarce [6]. Moreover, the topic is gaining importance because some authors see this technology as a more sustainable option for processing heavy crude oil [18,19].
Figure 3 shows the documents registered by country of origin, it should be noted that this graph also takes into account documents generated since 1970. The United States, China and the United Kingdom lead the intellectual production in this area. It is worth mentioning that, for the economies of the first two countries, the upgrading of heavy oil is strategic. In contrast, for the United Kingdom, the application of technology is being developed more widely than in other industrial sectors.
The scope of this work is limited to experimental studies, empirical evidence, and reviews related to developments applicable to surface operations. The experiments aimed applying the technology in oilfields, for Enhanced Oil Recovery purposes, were excluded, since they correspond to another branch of the development of this technology, addressed by diverse authors [18, 19, 20, 21]. Simulations were also excluded, since they are studied extensively by Niazi et al. [22, 23]
III. Results
This section presents the results of the literature review, organized in two main blocks: the first one explains the general facts of ultrasound applied to liquids and the acoustic cavitation phenomena. The second one describes the application of acoustic cavitation in heavy crude oil, and the experimental findings of the various authors.
A. Ultrasound and Acoustic Cavitation
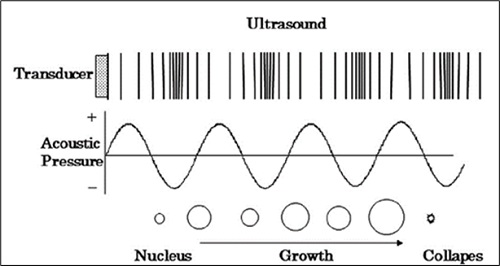
Applied to heavy crude oil, the phenomenon of acoustic cavitation triggers chemical reactions that break down the high molecular weight compounds associated with its high viscosity [24]. The cavitation phenomenon consists of the formation, growth and violent collapse of bubbles in a liquid. According to Streeter [25], these bubbles are formed in regions where the pressure is reduced and equals the vapor pressure. For this to occur, the concentration of energy within the fluid is required [26].
Acoustic cavitation occurs when ultrasound waves are propagated in a fluid [27]. As the longitudinal waves travel at a certain oscillation frequency, they induce molecular vibration and create expansion and compression cycles in the liquid particles [28], which is reflected in pressure oscillations (high and low pressures). In the areas where low pressures are created, there is a greater probability that cavitation bubbles will form. These bubbles absorb energy from the waves and increase their size until they lose the capacity to absorb energy efficiently [29]. Consequently, the surrounding liquid exerts a violent compression, because the external pressure is much higher, which produces an implosion of the bubble [24]. This implosion is what generates ideal conditions of temperature and pressure to favor chemical reactions [30,31]. Cavitation bubbles release energy in a localized way through the emission of shock waves. During the short time that the collapse lasts, considerably high temperatures and pressures are reached [25, 29, 30, 32]. Figure 4 shows the graphical representation of the acoustic phenomenon related to the dynamics of cavitation bubbles.
Several effects can be obtained with acoustic cavitation, including sonoluminescence and increased chemical activity in the solution [34]. The chemical activity increases due to the formation of new chemical species that are relatively stable, can diffuse in the solution, and can create chemical effects [24]. Another effect is the formation of free radicals in or around the bubbles, originated by the thermal decomposition of the molecules [33]. There are also significant physical effects, such as microacoustic flows (microstreaming), micro jets, micro currents and shock waves [35]. The mentioned effects can accelerate the mixing on a molecular scale and, consequently, favor the chemical reactions [36].
Acoustic cavitation can be used in various industrial processes, for example: in welding, destruction of cell walls, and dispersion of solids in liquids [36, 37]. However, the description of other applications is not covered in this article.
B. Effect of Ultrasound in Heavy Crude
This section describes the physicochemical phenomenon of the upgrading of heavy crude oil subjected to ultrasound. Extreme temperatures and pressures can cause physical and chemical changes that are evident in the rupture of high-molecular-weight molecules, transforming them into other substances of lower weight (light fractions) [24]. The increase in the proportion of lower weight molecules contributes to the reduction of viscosity. The breaking of heavy oil fractions with ultrasound follows the mechanisms of free radicals, similar to thermal cracking [38], then, hydrocarbon bonds are broken due to the energy released in the collapse of cavitation bubbles [39]. Regarding the chemical compounds that undergo conversion, it has been identified that the asphaltenes are reduced by the action of ultrasound [40]. It should be noted that these compounds are responsible for the high viscosity of heavy crudes. Other components for which there is evidence of degradation are resins, saturated, aromatic and naphthenic hydrocarbons [41].
The chemical process works as follows: after the collapse of the bubbles, free radicals are formed, which initiate a series of chain reactions [42]. Free radicals have a very aggressive reaction power, capable of degrading large and complex molecules. Their reactivity is due to the presence of an unpaired electron [43], and, initially, they originate from water associated with crude oil [44]. Since the energy released in cavitation and the extreme temperature favor the hemolytic rupture of water molecules, two free radicals are formed: the hydrogen ion (H+) and the hydroxyl radical (OH-), which are highly reactive. Subsequently, the C-C bonds belonging to saturated hydrocarbons (alkanes) are broken, from which additional primary radicals are formed, as well as secondary radicals, which react to form shorter chain alkenes and gaseous substances such as H2, CH4, and C2H4 [44]. After this, large molecules such as resins and asphaltenes begin to break down. Part of the asphaltenes is converted into gas oil and another into resins; later, the resin fraction formed is converted into gas oil, which corresponds to the saturated fraction of newly formed light compounds [38].
Physical phenomena also occur. Ultrasound induces the change in the colloidal structure of hydrocarbons, in particular, it releases the low-molecular-weight compounds associated with the structure of asphaltenes; in addition, the effects of cavitation weaken intermolecular interactions and, consequently, viscosity is reduced [45]. It has also been shown that the intermolecular association of asphaltenes is reduced under the effects of acoustic vibrations [38].
Once the phenomenon applied to crude oil has been described, the findings of various authors, who have carried out experimental and review work on this technology applied to the upgrading of crude oil, are described below.
The experimental Sonochemistry advances were relevant to provide foundation to the application of this technique to oil. Initially Suslick [24] investigated the effects of Sonochemistry on organic liquids and established that, if the vapor pressure is low enough to allow the effective collapse of the bubbles, almost all organic liquids will generate free radicals when subjected to ultrasound [24]. Figure 5 illustrates the birth, growth and collapse of cavitation bubbles, and also the rupture of high-molecular-weight hydrocarbon bonds.
Traditionally, this technology has been tried with the residues of the vacuum bottoms of refining towers, but it is currently being explored in heavy crudes to improve their rheological properties and commercial value [38]. Sadeghi et al [47] investigated the recovery of bitumen from the Athabasca (Alberta-Canada) oil sands, through acoustic cavitation using an ultrasonic bath (UB), also applying an aqueous alkaline sodium-silicate solution. The Ramsbottom carbon residue (RCR) of the bitumen recovered through acoustic cavitation (523 ºC+) was 12% compared to 22% RCR of the bitumen obtained by hot water process (Syncrude). The gravity of the bitumen recovered after acoustic cavitation was 15ºAPI compared to 8ºAPI of the bitumen in Athabasca sands.
On the other hand, Lin and Yen [38] postulated that the intermolecular association of asphaltenes is reduced under the effects of ultrasound. They identified asphaltenes degradation at room temperature and pressure. It was also verified that gas oil and resins can be obtained from asphaltenes; in addition, the resin fraction obtained can be converted into gas oil. On the other hand, it was found that the addition of surfactant avoids the agglomeration of asphaltenes and increases their conversion to lighter fractions, because they become easier when they are in emulsion, which is achieved by applying surfactants. Asphaltenes were reduced by 8% in a treatment time of 60 min. With the combination of acoustic cavitation, surfactant and a hydrogen donor (H2O2), Lin and Yen achieved a reduction of 35%, in an interval of 15 min [10].
Chakma and Berruti [48] presented the first study on the effect of ultrasonic vibrations on the viscosity of extra-heavy crudes, particularly in Athabasca bitumen and bitumen-solvent mixtures for different periods of time (10, 30, 60 min). They reported that pure bitumen presented a viscosity reduction of 12%, while in the bitumen-toluene mixtures it was reduced by 4% [48].
Lin and Yen [38] determined that cracking reactions form light fractions, while the water associated with the crude oil breaks down into free radicals that supply the hydrogen needed for the reaction. Years later, Cataldo [41] analyzed other oil fractions and suggested that both, cracking and pyrolysis of aromatic and naphthenic hydrocarbons, are possible using ultrasound. In his experiments he observed benzene and toluene aromatic rings become acetylene and other smaller fractions.
Gopinath et al. [49] investigated in detail the effects of ultrasonic treatment on the degradation of heavy gas oil (HGO) from the Athabasca bitumen without the use of additives. The authors identified low-volatility light hydrocarbons during the treatment, such as methane, ethane, ethylene, and propylene. This study reported maximum conversions of nitrogen and sulfur of 11% and 7%, respectively, and a reduction of 5% in viscosity, approximately.
Nesterenko and Berlizov [39] demonstrated that the energy released in the collapse of the cavitation bubbles breaks the hydrocarbon bonds of the oil compound molecules. They found that hydrocarbons with a molecular weight range of 100-300 g/mol are fragmented. Later, the experiments of Sawarkar et al. [40] and Kaushik [50] corroborated the reduction of asphaltenes in heavy crudes that were subjected to ultrasound. Sawarkar et al. [40] evaluated three different vacuum residues. They used an acoustic cavitation reactor to study the influence of reaction times from 15 to 120 minutes at room temperature and pressure. They also evaluated and compared the UH probe and UB ultrasonic bath systems, where the ultrasound probe showed better performance. The study reported a reduction in asphaltene content in the range of 30% to 59%, for the 60-minute irradiation time. Figure 6 shows the two main types of mechanisms for introducing ultrasonic energy into liquids.
Kuashik et al. [50] conducted experiments with residues from the vacuum bottom towers, which have a very high viscosity, similar to that of heavy oil. They tested acoustic cavitation for different reaction times (15 to 90 min) and with different probe diameters, at room pressure and temperature, and compared the effect of surfactants and the application of ultrasound, analyzing samples with surfactant without it. They concluded that there was not a significant difference due to the variation of the probe diameter; however, there was a substantial reduction of asphaltene in the presence of surfactant, of around 48%. On the other hand, without the application of surfactant, a reduction of 40% was achieved [50].
The ultrasound and a hydrogen donor have a synergistic effect on the process of improving vacuum bottoms. Yang et al [45] identified changes in the colloidal structure of hydrocarbons, the release of low molecular weight compounds associated with asphaltene structure, and observed that the effects of cavitation weaken intermolecular interactions and, consequently, viscosity is reduced. In their experiments, they selected Tetralin as a hydrogen donor; besides donating electrons, it has the ability to reduce the viscosity of vacuum bottom residues. In the absence of a hydrogen donor, the viscosity of the vacuum bottoms was reduced by 11 %. On the other hand, without ultrasound, but in the presence of Tetralin, the reduction was of 31%, and with the combination of ultrasound plus Tetralin, viscosity was reduced by 39%. The synergistic effect of ultrasound plus Tetralin allows the stabilization of the viscosity of the product, a higher percentage of light compounds, lower density, and a pour point lower than that obtained when these technologies are applied separately [45].
Mousavi et al. [51] studied the effect of ultrasound on the rheological properties of asphalt crudes for different time intervals (5-240 min). The authors observed an increase in the viscosity and the in the value of the effort of the elastic limit for the treated samples. Additionally, rheological analyses indicated that the ratio (viscous module values/elastic module values) is lower as the treatment interval is increased, which implies that the crude oil behaves in a more elastic manner, thus, making it more difficult to manipulate.
Diaz et al [10] evaluated the efficiency of ultrasound treatment on the viscosity of heavy crude oil from the eastern Colombian plains. The study evaluated a continuous flow system, analyzing the influence of the treatment temperature (308 and 319 K), exposure time (5.66 and 16.98 s) and sonic intensity (170-250 and 400-680 kW/m2) on viscosity. The study revealed that temperature has no significant effect on reducing viscosity. It was also found that the increase in exposure time favors the reduction of viscosity, while the sonic intensity has a favorable or unfavorable effect depending on the magnitude of exposure time [10]. The percentage of viscosity reduction that was achieved was 1.5%.
Xu et al. [52] studied the effects of ultrasound waves on a dilution of diesel, on extra-heavy oil from Venezuela, and on extra-heavy Fengcheng oil from China. They were unable to reduce viscosity in diluted diesel and extra-heavy crude oil from Venezuela, because ultrasound waves accelerate the dissolution of heavy components in diesel, especially asphaltenes. However, a viscosity reduction of more than 25% was achieved for the crude/water emulsion of the extra-heavy Fengcheng crude oil. It was also determined that 30% less viscosity-reducing chemicals are required [52].
Mullakaev et al. [53] studied the effects of ultrasound treatment on the temperature and viscosity properties of various types of crude oil with different composition. They concluded that the effectiveness of the ultrasound depends on the main group that comprises the crude and the treatment time. Crudes with low kerosene and high resin, tar and asphalt content tend to reduce their viscosity significantly, as does the pour point. In addition, efficiency increases with increasing exposure time. For crudes with high alkane content the treatment was not effective and an increase in the radiation time produced an increase in viscosity. The authors attribute this finding to a phenomenon of intensified crystallization of high molecular weight alkanes [53].
Salehzadeh et al. [54] conducted an experimental study of the effect of ultrasound on the aggregation and deposition kinetics of asphaltenes. They concluded that the sediments and the decantation rate of these substances are reduced, and that ultrasound allows to reduce the asphaltene aggregates that will finally have an effect on viscosity [54].
Askarian et al. [55] evaluated the effect of the presence of hydrogen donors in combination with metal nanoparticles, where they achieved a 20% viscosity reduction. Avvaru et al. [56] show how this technology can be applied to several processes related to the handling of heavy oil and explain the theoretical mechanisms of its operation.
Aliev et al. [57] mention the importance of heavy oil as a relevant energy source, they discuss the current difficulties in processing it and analyze various technologies. They focus on the description of ultrasound as an attractive alternative to improve the physicochemical characteristics of heavy crude oil. Montes et al. [58] conduct experiments to reduce the viscosity of heavy oil by applying ultrasound, a nickel-based nanoparticle catalyst, and water as a hydrogen donor. They achieved a viscosity reduction ranging from 44% to 16%.
Sawarkar [59] presents a systematic review of heavy oil upgrading techniques based on the cavitation process. He highlights the few advances in the field of acoustic cavitation, as well as the importance that technologies have nowadays, and the need to develop them for their application to heavy oil. Cui et al. [60] studied the structural changes of the essential components of Tahe crude oil, when it is treated with acoustic cavitation and a nickel catalyst. They concluded that cavitation affects the crystalline structure of the waxes or kerosenes that comprise the crude, which has implications on the reduction of viscosity [60]. The following table consolidates the results of some authors, showing the studied variables and the achieved results.
IV. Discussion
The studies found show that acoustic cavitation has effects on crude oil and other unconventional hydrocarbons, such as Athabasca sands and bitumen, gas oils and vacuum residues. Most of the research has been carried out with Ultrasonic Horn technology or ultrasound probes, which is more effective, as reported by Sawarkar et al. [40]. Regarding the studied variables, it can be said that there is a great dispersion of results and different methodological approaches, some of which study the reduction of asphaltenes, others test with distillation tests, while others consider API, sulfur content and viscosity [63]. With regard to viscosity, degrees of reduction have been reported in a wide range from very low percentages close to 1.5%, like the case of Diaz et al. [10], to relatively high percentages of 60%, as achieved by Mullakaev et al.[53]. However, it is worth mentioning that the reduction of asphaltenes with the use of acoustic cavitation has been soundly proven.
Many of the studies use additives to improve the performance of the process, reporting different types of surfactants and hydrogen donors; however, it is common for the selection criteria of these additives to be omitted in these studies. Only Lin and Yen [38] show a systematic approach to surfactant evaluation. Dependence on the use of surfactants for processing is a major disadvantage because of the increased costs when scaled to an industrial size.
The experimental studies analyzed could be classified in two: those that obtained favorable results and those that did not achieve significant reductions. However, the authors express convergent views regarding the technology analyzed, as they all recognize the opportunities offered by ultrasound, but point out the technical challenges that must be overcome today. It is important to note that the authors who did not achieve promising results usually give recommendations for future trials, and identify or explain the cause of the findings, but not one of them rules out this technology.
V. Conclusions
Ultrasound is an emerging technology that can be used in oil production operations. Achieving a significant reduction in viscosity at this point in the oil chain is very important, because it will reduce costs and operational difficulties in pipeline transportation and in refineries.
There are promising results at laboratory level, but without industrial application so far, partly because of the difficulties of scaling, and because all studies are exploratory, yet none point to the systematic development of the technology.
Another difficulty is that there is not yet uniformity regarding the degree of viscosity reduction that can be achieved, because the range of variation observed in the reported publications is very wide, and, thus, it is difficult to predict the result of the process. Moreover, some authors report contradictory results, in which, instead of achieving a decrease in viscosity, they report an increase [9, 47].
Since many studies have been conducted with additives to increase the percentage of viscosity reduction, the true potential of acoustic cavitation without the use of these substances is unknown. Determining the performance without additives would minimize the demand for these chemicals, which would optimize costs.