Introduction
Chronic kidney disease (CKD) is a pathology that affects 10% of the global population with high impact and mortality1. Living kidney transplant is the best therapeutic option for patients with CKD due to higher graft survival, lower cold ischemia time, and decreased risk of delayed graft function (DGF). The incidence of DGF in recipients with a cadaveric donor is 21.8% versus 3.5% in recipients with living donors2.
Some of the general medical aspects of laparoscopic living donor nephrectomy (LDN) have been evaluated in different publications3,5. For example, the use of mannitol during the living donor laparoscopic nephrectomy is associated with increasing the renal blood flow, decreasing endothelial swelling, and mitigation of free oxygen radicals3,6
Mannitol (C6H8(OH)6) is an alcohol that releases prostaglandins leading to renal vasodilatation and increased diuresis. The literature reported that these effects contribute to the protection of renal injury and the preservation of kidney function7. In kidney transplantation, the living donor kidney is susceptible to ischemic reperfusion insult while clamping the donor's renal artery and flushing it with a cooled preservation solution. The intraoperative mannitol in LDN results in the mitigation of this ischemic injury and the reduction of tubular cell swelling to prevent acute tubular necrosis (ATN) and DGF in kidney transplant recipients6. However, some studies assessing mannitol have contradictory findings4,8.
DGF is determined as an indication for dialysis in the 1st week after kidney transplantation and is associated with post-transplant oliguria, a higher risk of acute rejection, and lower graft survival9. The DFG increases the risk of acute rejection by 20-40%10. Some studies published that the administration of mannitol declined the incidence of acute rejection11.
In Latin America, there are no studies with evidence that allow extrapolating data about the administration of mannitol during LDN and its benefits in kidney transplant. This research aimed to evaluate the administration of mannitol during the hand-assisted LDN (HALDN) and the incidence of DGF. Furthermore, this study aimed to achieve two specific objectives. First, the clinical and sociodemographic characteristics of donors and transplant recipients will be described within the timeframe of January 2015 to December 2019. Second, we will conduct a comparative analysis of post-operative outcomes, focusing on different follow-up times, specifically between recipients who received mannitol and those who did not receive mannitol. Our hypothesis was to examine the differences following the administration of mannitol since according to multiple scientific studies, the administration of mannitol has been demonstrated to reduce the occurrence of acute rejection. In addition, we are interested in determining whether these differences are statistically significant.
Methodology
Study design
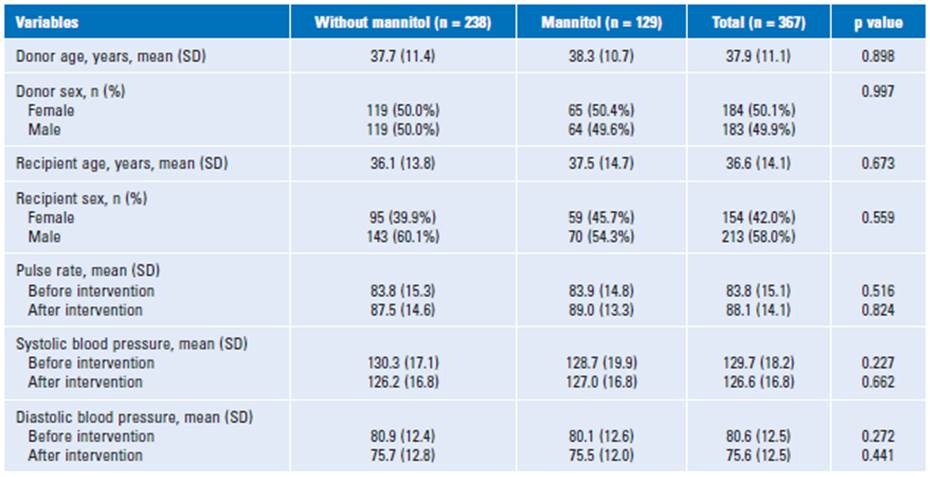
A retrospective cohort observational study was performed including living kidney donors (LKD) and their recipients during the period from January 2015 to September 2019 at Colombiana de Trasplantes. A non-probabilistic convenience sampling method was employed for the non-random allocation of the intervention. We obtained through Stata 14 a sample size of 124 (62 per group). Our transplant center performs 44% of the total living donor kidney transplant activity in Colombia12. During the study period, 367 patient recipients and their donors were assessed. Electronic clinical records were reviewed for all study populations. Demographic data and clinical outcomes were collected from institutional medical records for our database.
Donor evaluation
All LKDs are evaluated by a multidisciplinary team and the Ethics Committee. Laboratory studies were performed to determine the suitability of the LKD. The LKD glomerular filtration rate is measured by 24-h urinary creatinine clearance. Computed tomography angiography was performed to identify the renal vascular anatomy. Our inclusion criteria for the donor were individuals aged over 18 years, approved by the Medical Board and Ethics Committee, and with donation dates falling between January 2015 and December 2019. As for the recipient, we included individuals aged over 18 years who received a kidney transplant from a living donor within the specified time frame. However, we excluded recipients who underwent auto-transplantation.
Recipient selection
The evaluation of kidney transplant candidates was performed by mental health, transplant nephrology, and transplant surgery. It is imperative that the kidney transplant benefits the recipient over the risks, the candidate tolerates the surgery, and the immunosuppression would not deteriorate or does not cause exacerbation of comorbidities.
Hand-assisted laparoscopic living donor nephrectomy
The HALDN was the technique of choice in our transplant group. Donors were positioned in a "flank-up" position. The HALDN requires a hand port, two trocars (5 and 12 mm), and a 30° video endoscope. The pneumoperitoneum had a flow rate of 400 cm/min and 15 mmHg of intra-abdominal pressure. The ultracision (HARMONIC® HD ultracision johnson 1000i) mobilized the colonic splenic flexure, and renal artery vessels and ureters were identified and dissected. Renal vessels were clamped using two large-size non-absorbable polymer ligating clips (Weck® Hem-o-lok®). The use of a 60 mm endovascular cutting stapler obtains a reasonable vessel length to mobilize and remove the left kidney. The kidney is immediately removed to minimize the warm ischemia time. The kidney was delivered through the hand port. A laparoscopic inspection was done to check for hemostasis and closure of the abdominal cavity.
Mannitol administration
The decision to administer mannitol was made based on the surgeon's clinical judgment and expertise, taking into consideration the existing scientific literature and evidence supporting its potential benefits in reducing the incidence of acute rejection. During HALDN, before renal artery clamping, a 30-min infusion of 20% mannitol was administered to all exposed patients, following the clinical practice guidelines.
Variables and measurements
The study included the following variables: age, sex, creatinine, DGF, urinary volume in 24 h, acute rejection at 3 months, and mortality at 3 months. In terms of the outcomes, DGF was defined as the requirement for dialysis within the first 10 days following transplantation. Acute rejection was identified based on biopsy findings using the BANFF criteria. Mortality was documented through clinical records. We employed standardized protocols for data collection, utilized reliable data sources, and conducted assessments at consistent time points.
Statistical analysis
Descriptive analyses reported the population demographics, and clinical data according to the nature of the variable and distribution. The data were displayed as frequencies and percentages to describe categorical variables. Central tendency and dispersion measures were used to describe quantitative variables. The study population was divided into two groups (with or without mannitol administration) comparing the main clinical outcomes. Bivariate analysis was performed to compare the main clinical outcomes (DGF, urinary volume in 24 h post-transplantation, acute rejection, and mortality after 3 months of kidney transplant) between the mannitol group and without mannitol group. p < 0.05 was accepted as statistically significant.
Analysis was performed using Software R version 4.0.3.
Results
Demographics and baseline characteristics
A total of 367 recipients and their donors were evaluated during the study period. Among those, 129 (35%) had the administration of mannitol. The mean age of donors was 37.9 ± 11.1 years. Approximately half of donors were female (50.1°%). The recipients had a mean age of 36.6 ± 14.1 years old, and most of the patients were male compared to the gender proportion (58% versus 42%). There were no significant differences in the bivariate analysis corresponding to age or gender either in LKD or recipients with or without administration of mannitol. Table 1 depicted demographics and baseline characteristics of LKD and recipients with or with-out mannitol use.
Clinical outcomes in recipients
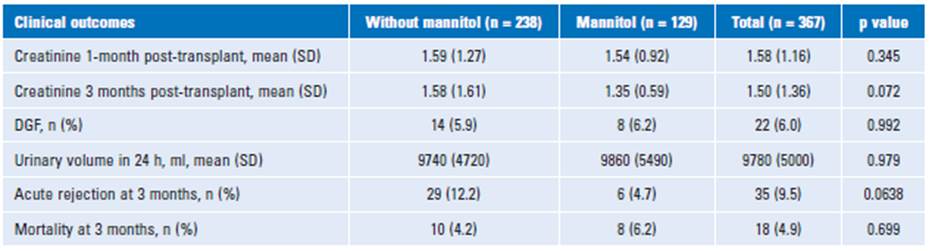
The incidence of DGF was 5.9% in the group without mannitol compared to 6.2% with mannitol (p = 0.99). The urinary volume (diuresis) in the first 24 h after the kidney transplant was de 9740 ± 4720 ml without mannitol versus 9860 ± 5490 ml with mannitol. No significant differences were found in diuresis and mortality between both groups (p = 0.97 and p = 0.69, respectively). The incidence of acute rejection had a trend in the difference between the groups (without mannitol 12.2% versus mannitol 4.7%) but was still not significant in the bivariate analysis (p= 0.06) (Table 2).
Serum creatinine after kidney transplant
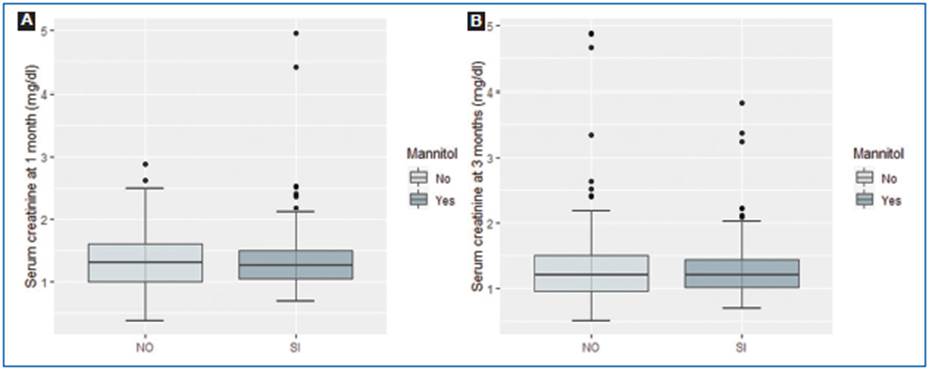
The serum creatinine mean was 1.59 ± 1.28 mg/dl without mannitol versus 1.54 ± 0.925 mg/dl with mannitol (p = 0.92) 1 month after kidney transplantation. At 3 months of follow-up, serum creatinine mean was 1.58 ± 1.61 mg/dl without mannitol compared to 1.35 ± 0.6 mg/dl with mannitol (p = 0.34) (Fig. 1).
Discussion
This is the first study in Colombia that sheds light on the role of mannitol administration, considering clinical and sociodemographic variables, as well as post-operative outcomes in our population. Mannitol is one of the main osmotic and diuretic medications used in kidney transplant15. The intraoperative administration of mannitol during the HALDN is associated with the prevention of DGF6 and acute kidney injury8 in LKD recipients. To the best of our knowledge, there are no publications about the assessment of the effect of mannitol in HALDN or kidney transplantation in Latin America. This study described the impact of intraoperative use of mannitol during HALDN in LKD and the incidence of DGF in kidney transplantation.
In our study, the demographic characteristics showed a gender distribution in LKD and recipients akin to the previous publications16.
At first glance, the main clinical outcomes analyzed for the recipients in this study without or with the use of mannitol were as follows: kidney function, the incidence of DGF, urinary volume after 24 h of a kidney transplant, the incidence of acute rejection, and mortality at 3 months of follow-up.
In the literature, the DGF is correlated with a higher risk of multiple post-transplant complications and less graft survival in kidney transplant recipients9,17.
In our findings, the incidence of DGF did not have a significant difference within the comparison groups similar to a study of 413 LKD with laparoscopic nephrectomy18. Andrews et al.6 evaluated varying degrees of ATN by performing an optical coherence tomography in LKD comparing mannitol infusion for 15 min versus 30 min in the renal tubules. They found a higher incidence of ATN in the group with mannitol infusion for 30 min than with mannitol infusion for 15 min. In kidney transplantation, a meta-analysis that included two studies with 569 LKD recipients described that the use of intraoperative mannitol decreased the incidence of DGF from 30-55% to 14-21% in the mannitol groups (p = 0.02)8.
Esfahani et al.4 published a random clinical trial with 60 LKD with or without intraoperative mannitol evaluating the effect of mannitol on diuresis and serum creatinine concentration. The outcome did not show significant differences between mannitol and placebo in a way similar to our results. On the contrary, the meta-analysis mentioned above found in a study with kidney transplant patients that those who received mannitol had higher diuresis than the control group. In parallel, serum creatinine showed heterogeneous results in this meta-analysis8.
On the other hand, the reduction in the incidence of acute rejection at 1 year of follow-up in the Williams et al.18 results was not significant with the administration of mannitol in LKD equivalent to our findings. In addition, a total of 90 kidney recipients were evaluated to determine the incidence of acute rejection with or without mannitol in kidney transplantation. In this report, there was no significant association between a lower risk of acute rejection and the use of mannitol8. Finally, there were no publications that evaluated mortality and intraoperative mannitol use in LKD or kidney transplant recipients.
The study has several limitations that should be acknowledged. First, selection bias may exist due to the lack of standardized criteria for participant inclusion, potentially affecting the generalizability of the findings. Second, subgroup comparisons might be challenging due to the heterogeneity within the subgroups. Third, the use of retrospective measurement introduces the possibility of measurement bias and data quality issues. Inaccuracies or missing information may affect the reliability of the results. Finally, confounding bias could be present as unmeasured factors associated with both the exposure and outcome may influence the observed associations. Given that this study was conducted in a single healthcare center, the ability to extrapolate the findings is considered limited, and further studies are needed to gain a better understanding of the studied phenomenon. Consequently, the study exhibits low external validity and highlights the need for additional research.
The study has several strengths that enhance its value and credibility. First, despite its retrospective design, it provides valuable insights and allows for the examination of associations and trends over time. Second, the comprehensive data collection approach utilized in the study, including data retrieval from a reliable database, ensures a robust dataset for analysis. Third, the longitudinal analysis enables the assessment of outcomes and exposures over multiple time points, enhancing the understanding of temporal relationships. In addition, the inclusion of relevant variables, such as kidney function, provides valuable clinical information and allows for an in-depth analysis of relevant outcomes. Finally, the study's retrospective design reflects real-world clinical practices, offering the potential for real-world application and insights into the effectiveness of interventions in routine healthcare settings. These strengths collectively contribute to the study's overall significance and strengthen the validity and applicability of its findings.