Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Colombiana de Ciencias Pecuarias
versão impressa ISSN 0120-0690
Rev Colom Cienc Pecua vol.26 no.2 Medellín abr./jun. 2013
ORIGINAL ARTICLES
Osmoregulation of juvenile marine goliath grouper (Epinephelus itajara) in low-salinity water¤
Osmorregulación de juveniles del mero guasa juvenil (Epinephelus itajara) en aguas de baja salinidad
Osmorregulação de juvenis do peixe garupa (Epinephelus itajara) em águas de baixa salinidade
Lury N García1, Ing Pesq, MSc; Clara L Sierra2, Biól; Jeiver Perez2, Biól; Frank Esquivel2, Biól; Frank A Chapman3*, Biól, MSc, PhD.
* Corresponding author: Frank A Chapman, University of Florida, Fisheries and Aquatic Sciences, 7922 NW 71st Street, Gainesville, Florida 32653, USA. e-mail: fchapman@ufl.edu
1 Programa de Tecnología en Acuicultura, Universidad del Pacífico, Av Simón Bolívar # 54A-10 Los Laureles, Buenaventura, Valle del Cauca, Colombia.
2 CVS, Calle 29 No. 2-43 Ed. Morindo, Córdoba, Colombia.
3 Fisheries and Aquatic Sciences, University of Florida, 7922 NW 71st Street, Gainesville, Florida 32653, USA.
(Received: December 15, 2011; accepted: November 15, 2012)
Summary
Background: goliath grouper (Epinephelus itajara) is an economically valuable marine species and an excellent candidate for domestication for aquaculture purposes. If this grouper can osmoregulate in lowsalinity water, its cultivation can provide socio-economic benefits, for both coastal communities and the mainland agricultural sector. Objective: to evaluate the osmoregulatory capacity of juvenile goliath grouper when exposed to low-salinity water. Methods: juvenile goliath grouper (Epinephelus itajara) were either directly or gradually transferred from seawater to freshwater to test osmoregulatory ability. Body weight was assessed during acclimation and blood samples were taken to measure total osmolality and electrolytes. Results: all fish survived the transfer to freshwater and were maintained for up to 12 days after termination of the acclimation trials which lasted 72 hours. Juvenile goliath grouper were hyposmotic (342-462 mosmol/kg) to seawater and hyperosmotic (272-292 mosmol/kg) to freshwater. The gills and kidneys were found to have principal roles in the osmoregulatory processes. Numerous chloride cells were found on superficial regions of the gill filament epithelium, most likely serving to eliminate the excess of electrolytes while in seawater. The kidneys had numerous nephrons to make urine and retain electrolytes while in freshwater. Conclusions: these observations lead to the conclusions that juvenile goliath grouper have the ability to osmoregulate in freshwater and should be considered a marine euryhaline species. Such adaptability opens for consideration the possibility that goliath grouper could be successfully farmed in brackish water or even in freshwater.
Key words: chloride cells, conservation aquaculture, euryhaline, nephrons, salinity tolerance.
Resumen
Antecedentes: el mero guasa Epinephelus itajara es una especie marina de gran valor comercial y un excelente candidato a domesticar con fines acuícolas. Si el mero guasa puede osmoregular en agua de baja salinidad, su cultivo puede proporcionar beneficios socio económicos, tanto para las comunidades costeras, como al sector agropecuario en tierra firme. Objetivo: evaluar el efecto en la osmoregulación de juveniles de mero guasa expuestos a aguas de baja salinidad. Métodos: juveniles de mero guasa mantenidos en agua de mar fueron transferidos directamente o de manera gradual a agua dulce para poner a prueba su capacidad osmorreguladora. Durante el proceso de aclimatación se les evaluó el peso corporal y se extrajo sangre para medir la osmolalidad total y electrolitos. Resultados: todos los peces sobrevivieron la transferencia al agua dulce y durante 12 días más, después de la finalización de los ensayos de aclimatación que tuvieron una duración de 72 horas. Juveniles de mero guasa fueron hiposmóticos (342-462 mosmol/kg) respecto al agua de mar e hiperosmóticos (272-292 mosmol/kg) respecto al agua dulce. La histología de branquias y riñones reveló que estos órganos son de gran importancia en los procesos osmorregulatorios. Un gran número de células de cloruro fueron localizadas como parte del epitelio de los filamentos branquiales; estas células trabajan para librar al cuerpo del exceso de electrolitos mientras los peces se encuentran en el mar. En el riñón se observaron numerosas nefronas y túbulos colectores para la formación de orina y retención de electrolitos; tejidos esenciales si estos peces permanecen en agua dulce. Conclusión: estas observaciones llevan a la conclusión de que los juveniles de mero guasa tienen la capacidad de osmorregular en agua dulce y debe ser considerada una especie marina eurihalina. Tal adaptabilidad supone la posibilidad de que el mero guasa podría ser cultivado en agua salobre o incluso en agua dulce.
Palabras clave: acuicultura de conservación, células de cloruro, eurihalino, nefronas, tolerancia a la salinidad.
Resumo
Antecedentes: o peixe garoupa Epinephelus itajara é uma espécie marinha de muito valor comercial a qual seria ótimo ter domesticada para sua produção industrial na aquicultura. Se o peixe garoupa pode osmoregular em água de baixa salinidade, sua cultura pode proporcionar benefícios socioeconômicos, tanto para as comunidades costeiras, quanto para o sector agrícola no interior do continente. Objetivo: avaliar a osmoregulação de juvenis do peixe garoupa expostos a águas de baixa salinidade. Métodos: juvenis do peixe Garoupa mantidos no mar foram transferidos direta ou gradualmente para água doce testando assim sua capacidade osmorregulatória. Durante o processo de aclimatização, foi avaliado o peso corporal e amostras de sangue foram coletadas para medir a osmolalidade total e alguns eletrólitos. Resultados: todos os peixes sobreviveram à transferência para água doce 12 dias mais após a conclusão dos estudos de aclimatação que se fizeram durante um período de 72 horas. Juvenis do peixe garupa foram hiposmoticos (342-462 mosmol/kg) com respeito à água marinha e hiperosmóticos (272-292 mosmol/kg) com respeito à água doce. Histologia das brânquias e os rins revelaram que estes órgãos são de grande importância nos processos de osmoregulaçao. Um grande número de células de cloreto foi localizado como parte do epitélio dos filamentos branquiais; estas células trabalham no organismo para livrar o corpo do excesso de eletrólitos enquanto os peixes estão no mar. Nos rins foram observados numerosos néfrons e ductos recoletores para a formação de urina e retenção de eletrólitos; tecidos essenciais no caso de que estes peixes permaneçam em água doce. Conclusão: estas observações levam à conclusão de que os juvenis do peixe garupa tem a capacidade de osmoregular em água doce e deve ser considerado uma espécie marinha eurialina. A adaptabilidade deste peixe em água doce supõe a possibilidade de que o peixe garupa poderia ser cultivado nesta água em criadouros no interior do continente.
Palavras chave: aqüicultura de conservação, células de cloreto, eurialina, néfrons, tolerância à salinidade.
Introduction
Aquaculture is one of the fastest-growing segments of agriculture, advancing worldwide at a rate of approximately 6.5 to 8.3 percent per year (FAO, 2010). However, unlike terrestrial animals, the process of domestication of many aquatic organisms remains in its infancy. Most commercially grown fish and shellfish species are primarily collected from the wild. Understanding biological functions, mainly reproduction, genetics, nutrition, health, and environmental physiology, are necessary in order to prioritize the selection of species for domestication. Knowledge of environmental physiology is necessary to determine optimal environmental conditions in order to achieve the best animal performance and stock health.
Groupers have traditionally been among the world's most valuable fishery commodities, especially in tropical countries, both for their meat and recreational value. They are also an important component of commercial and sport fisheries in both the Atlantic and Pacific coasts of Colombia. The goliath grouper Epinephelus itajara, or mero guasa as it is known in Colombia, is the largest grouper in the Western Atlantic Ocean, reaching 455 kg and over 2.5 m in body size (Bullock et al., 1992). Goliath grouper, however, has been over- fished throughout much of its natural range and many populations have become threatened to the point of extinction. For example, in Brazil and the USA the species is legally protected from fishing and in Colombia it is listed in the Red Book of threatened marine species—those requiring urgent and efficient protective measures (Mejía and Acero, 2002; Hostim-Silva 2005; NMFS, 2006). This species is also listed as critically endangered by the International Union for Conservation of Nature and Natural Resources (IUCN, 2011).
The conservation and restoration efforts for goliath grouper will ultimately depend on appropriate management rules and aquaculture management practices within the fisheries. Aquaculture conservation programs can produce juveniles of the species that can be used to augment populations in the wild. Development of commercial ventures for the cultivation of goliath grouper can greatly reduce pressure on wild populations. Farming goliath grouper can also be a beneficial and valuable agricultural alternative in rural coastal areas. Preliminary studies documented that wild- caught goliath grouper juveniles acclimated well to captivity and grew fairly quickly when properly fed (Torossi, 1982; Cervigon, 1983; Botero and Ospina, 2003). Furthermore, ecological investigations indicated goliath grouper is a relatively hardy species since the juveniles live primarily within the mangrove biome characterized by extreme fluctuations in water quality (Smith, 1971; Bullock et al., 1992; Sadovy and Eklund, 1999; Eklund, 2005; Frias-Torres, 2006; Koenig et al., 2007).
The objective of this study was to determine if juvenile goliath grouper can osmoregulate in water that is less concentrated in solutes than their own body fluids or that of their marine environment. Temperature, salinity, dissolved oxygen, dissolved salts and ions, ammonia, and pH are probably the main physical and chemical factors that affect or limit the distribution of fishes in nature as well as their optimal performance under culture conditions (Moyle and Cech, 1982; Timmons et al., 2002). Although primarily a marine species, goliath grouper may have the osmoregulatory capacity to thrive in very low salinity water or even freshwater. If goliath grouper can osmoregulate in low salinity water, its aquaculture can become a technically and economically viable proposition in inland areas, especially for intensive farming operations with traditional land-based production systems. As far as we know, this is the first study of its kind carried out on the species.
Materials and methods
Juvenile goliath groupers were collected in the coastal waters of Puerto Cispatá in the Atlantic coast of Colombia. They were fished in shallow water over sand flats with baited hooks and in mangrove sites with dip nets and bare hands as the fish hid within the cavity of rubber tires formerly placed there to create hiding places for them. After collection, the fish were immediately transported to a fish camp on stilts where they were held in cages constantly exposed to seawater (26 to 32 parts per thousand [ppt] at 28 to 32 ºC), and fed twice daily with fresh pieces of cut baitfish until the experiment began. Immediately prior to each trial, groups of fish were removed from the cages and transferred to a laboratory where they were kept in a ceramic tiled tank (3.0 x 1.2 x 0.6 m; 2,462 L) that served as an acclimation system and container for the trials.
A continuous supply of filtered and aerated seawater and freshwater was available throughout the study. Fish were maintained at 28 to 29 ºC in the tank and exposed to natural light. Fish were held in this tank without feed for 24 h until trials commenced. For the experimental trials, the juvenile grouper (n= 50; total length= 29.3 ± 2.6 cm, standard length= 23.6 ± 2.2 cm, and total weight= 424.9 ± 125.9 g) were randomly selected, divided into experimental groups, and transferred from the ceramic tiled tank to circular, 800 L plastic water storage tanks that served as experimental containers. Each tank was fitted with a submersible water pump to achieve constant water circulation and aeration. Water in the tanks was maintained at ambient temperature (28 to 29 ºC), and exchanged twice daily when the fish holding period was extended beyond 24 h. Water quality in all tanks was monitored twice daily for total ammonianitrogen using a portable MI405 ammonia detector (Milwaukee-Martini, Rocky Mount, NC, USA).
Temperature, salinity, and dissolved oxygen were measured using a YSI 85 handheld multiparameter instrument (YSI, Yellow Springs, OH, USA), while pH was measured using a digital ISFET meter (IQ Scientific, San Diego, CA, USA). Fish were acclimated to the conditions of the experimental tanks for 24 h before each trial began. Fish were not fed 24 h before experiments or during trials in order to reduce interference with blood tests and maintain water quality.
The experiments were divided into two groups. Group 1: the experiment involved measuring osmolality and blood electrolytes of juvenile goliath groupers (n= 20; 24.4 ± 2.4 cm standard length, and 401.0 ± 103.9 g) held in natural seawater (SW) in which they normally live, and those acclimated to freshwater (FW). The fish were acclimated to FW following a protocol we developed and successfully tested. Briefly, the fish (n= 6; 24.7 ± 0.7 cm standard length, and 565 ± 63.4 g) were gradually transferred over a period of 3 to 4 days from full strength natural SW to FW. Initial salinity of 29 ppt was reduced to 20 ppt. After 12 h salinity was reduced to 15 ppt, and after 24 h to 8 ppt. The fish were held at 8 ppt for 48 h then transferred directly to FW. The original salinity was reduced by dilution from one concentration to the next over a 1 h period. A salinity refractometer was used to determine concentration.
Group 2: to determine osmoregulatory capability, fish (n= 24; 22.9 ± 2.1 cm standard length, and 436.6 ± 138.0 g) were transferred directly from natural SW (29 ppt) to FW (<1 ppt). Three fish were weighed and blood sampled at the beginning (0 h), then 4.5, 9, 24, 30, 48, 64, and 72 h into the experiment. The fish were weighed in a tarred plastic pitcher to determine changes in total body weight (TW ± 0.1 g) and were measured for standard length (SL) and total length (TL) on a calibrated board (± 1 mm). To draw blood, fish were killed by decapitation followed by quickly cutting the caudal peduncle thereafter. Blood from individual fish was collected directly from the bleeding caudal vessels into heparinized Caraway capillary tubes. Osmolality (mosmol/ kg), sodium (Na+), potassium (K+), and chloride (Cl-) were measured in fresh whole blood directly from the capillary tube using a Wescor 5500 vapor pressure osmometer (Wescor, Logan, UT, USA) and a Medica Easy Electrolytes analyzer (Medica, Bedford, MA, USA), respectively. Pieces of gill and kidney were removed from five of the fish kept in natural SW and five of the fish acclimated to FW.
Tissue samples were transferred to 10% neutral buffered formalin for preservation, embedded in paraffin, sectioned, and stained with hematoxylin and eosin, using standard histology methods (Bancroft and Gamble, 2002); some of the gill tissue samples were further stained with toluidine blue to identify chloride cells. Sections (4-5 μm) of gill filaments and kidneys were examined using light microscopy to identify general structural features of the tissues, presence of chloride cells, and to determine if they displayed gross morphological effects of FW. Data were organized in Excel application spreadsheets (Microsoft, Redmond, WA, USA) and analyzed using JMP statistical software (SAS, Cary, NC, USA). Comparisons between means (mean ± standard deviation) were made with the t-test and analysis of variance procedure was conducted with the Tukey's multiple comparison post test at a 95% level of confidence.
Results
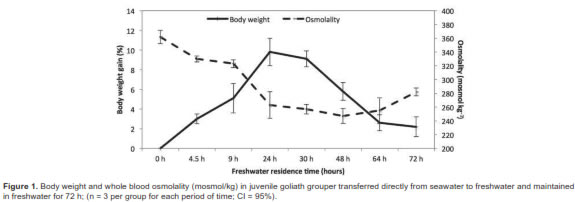
All juvenile fish survived in SW and FW with no signs of distress throughout the entirety of the experiment. They were maintained intermittently from a minimum of 72 h up to 12 d. The SW adapted juvenile fish had blood osmolalities between 342 and 462 mosmol/kg, and sodium ion concentrations around 186 to 189 mmol/L, chloride levels above 150 mmol/L, and 5.0-5.9 mmol/L of potassium (Table 1). Once SW adapted fish were transferred directly to FW there was a rapid weight gain (up to approximately 10%), and a significant tendency of blood osmolality to decline during the following 24 h (Figure 1).
There was also a noticeable decrease in ion concentrations (Figure 2). At 30 h individual fish stopped gaining weight and there were apparent concomitant ionic readjustments. By 48 h blood osmolality had leveled off while ions began to be retained. Restoration of hydromineral balance was apparent after 72 h. In juvenile fish kept in FW, the experimental osmolality values ranged between 272 and 292 mosmol/kg, for sodium 147 to 159 mmol/L, chloride 117 to 125 mmol/L, and 3.4 to 4.9 mmol/L for potassium (Table 1). Overall, the FW adapted fish had slight but significantly lower solute concentrations and osmolality values than those in SW (Table 1). Although these fish were not sampled again, they were maintained in FW for 12 additional days without apparent ill effects.
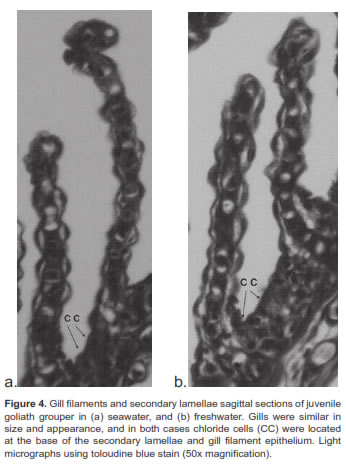
Chloride or mitochondrial rich cells (MRC) were primarily located at the base of the gill filament in juvenile goliath grouper (Figure 3). In both SW and FW acclimated fish, the MRC were agglomerated at the base of the gill filament epithelium and were difficult to distinguish in the lamellae (Figure 4). However, during acclimation from SW to FW we did not detect changes in the size or number of MRC in the gill filament. Histologic examination of renal tissue from juvenile goliath grouper indicated they possessed a well differentiated kidney structure. The renal corpuscle, the renal tubule, and the collecting duct system of the kidney were clearly distinguished by light microscopy (Figure 5). No evident differences were observed histologically between the kidneys of SW and FW acclimated fish (Figure 6).
Discussion
Juvenile goliath grouper certainly has the basic anatomical structures (gill and kidney) and active mechanism for regulating body water and ion concentrations to adapt to low salinity water. Blood osmolality values were little affected, if at all, by the external medium osmolality as they remained close to one third of normal seawater (hyposmotic) and above that of freshwater (hyperosmotic). Therefore, rather than stenohaline, goliath grouper may be considered euryhaline. Although there were statistically significant differences for mean whole blood osmolarities and ion concentrations in fish kept in saltwater with respect to those in freshwater, all levels fell within the expected normal osmolarity ranges and ion concentrations found in blood of marine and freshwater adapted fish.
It was apparent that during the 72 h acclimation period juvenile goliath grouper underwent significant variations in water balance, resulting in considerable alterations in osmolality and most ionic concentrations. Body weight gain was probably correlated to changes in bulk water absorption or osmotic water gain as has been well documented in most animals (Evans, 2009). Likewise, excess water diluted extracellular osmolality as reflected in the decreased plasma osmolality and ion concentration levels observed.
However, after 48 h juvenile goliath grouper appeared to stabilize or compensate for osmotic water gain and diffusional ion loss, and its recovery was initiated. Interestingly, potassium ion concentration was very well sustained throughout the period of acclimation. However, the highest concentration of potassium in the body of animals is inside the cells (intracellular fluid compartment, ICF), instead of outside the cells (extracellular fluid compartment, ECF), where the other principal ions are contained (Tasker, 1980; Evans, 2009). Since cell volume control is primarily regulated by the gain or loss of primary ions such as Na+, and Cl- in the ECF, and K+ together with hundreds of millimolar molecules (organic osmolytes) within the ICF, the effect of external osmotic changes at the intracellular level can lag behind relative to electrolyte uptake in the ECF (Tasker, 1980; Choe and Strange, 2009). The 72 h allocated to the acclimation experiments was sufficient time for the fish to acclimate to low salinity water. It is known that salinity tolerance of fish can be determined using standard or modified LC50 acute toxicity testing methods (Kefford et al., 2004). Fish exposed beyond the tolerated salinity range died within 48 to 72 h, but those fish that tolerated a wide salinity range had a mean survival of at least 90% at 72 h (Kefford et al., 2004). No juvenile goliath grouper died during the experiments as they gradually acclimated from seawater to freshwater. As predicted, indicators of osmoregulatory capacity in the juvenile goliath grouper could be measured within 48 and 72 h.
Most studies indicate that the principal osmoregulatory organs in fish are the gills, kidney, and gut (Evans and Claiborne, 2009). Microscopic examination of gills and kidney tissues of SW and FW acclimated juvenile goliath grouper revealed they had all the characteristics of well-developed organs to adapt to water of different salinity. Chloride cells or MRC were observed along the filamental epithelium of the gill similar to that of other marine fish species. Given the ionic gradient across the gill in SW, chloride cells or MRC have been identified to function as the main organ in salt excretion (Evans and Claiborne, 2009). However, no apparent differences in gross histology of gill tissues (e.g., number of chloride cells; p>0.05) were observed between SW and FW acclimated fish. While behavioral and physiological changes at osmoregulatory compensation tend to occur between 48 and 72 h, identifiable structural or histological changes such as proliferation or disruption of chloride cells may become visible later. For example, the number of chloride cells in the gill filament or lamellae in anadromous salmon were observed to be continuously replaced by newly-differentiated cells, but, depending on their location, the total numbers observed could remain constant (Uchida and Kaneko, 1996). Thus, changes in total number of chloride cells in gills of juvenile goliath grouper may become apparent only after wide differences between net turnover rate and net synthesis rate or chloride cell degradation take place, which could take longer than the 3 d duration of this experiment. In contrast to the function of the gill in SW, the kidney may play the dominant role in the osmoregulatory mechanism of juvenile goliath grouper in FW to compensate for osmotic water gain and diffusional ion loss. Juvenile goliath grouper kidneys possessed well-organized renal corpuscles, tubules for reabsorption of electrolytes, and a collecting duct system for eliminating large quantities of dilute urine, typical of kidney tissue of freshwater species. Although not evaluated in this study, the gut could also play a significant role in juvenile goliath grouper osmotic and ionic regulation as it does in most other fish. For example, SW fish must drink seawater to offset osmotic and renal loss of fluid while in FW fish the gut serves as site for Cl- and K+ reabsorption (Evans and Claiborne, 2009).
The osmoregulatory capability of juvenile goliath grouper may explain their abundant presence in estuaries and the extent of their euryhalinity. Such osmoregulatory capability also indicates the potential for growing the species not only in marine environments but also in brackish and freshwater conditions. For many years, several grouper species have been cultivated on a commercial scale primarily in China, Japan, Taiwan, Southeast Asia, and the Middle East, but such practice relies primarily on the collection of juveniles from the wild followed by growth in captivity to reach marketable size (Tupper et al., 2008). Sustainable aquaculture practices, however, must depend on a reliable supply of juvenile grouper raised in hatcheries and obtained from domestic stocks. One of the ultimate goals of this study was to begin investigations on the possibility of raising goliath grouper in brackish or freshwater conditions. Aquaculture of marine species in coastal areas or inland is an attractive proposition, especially for reducing the inherent risks of offshore cage culture regarding activities such as maintenance and repair of production structures, husbandry of the stock, disease transmission to natural stocks, pollution of the benthic community, and genetic and ecological impacts due to escapement. Conflicts of ownership, public perception, and technical issues in site selection are the main difficulties of offshore aquaculture facilities. One of the benefits identified with the culture of marine grouper species in lower salinity water was an observed reduction in the incidence of disease; however, the studied species succumbed in lower than 5 ppt salinities (Woo and Wu, 1982; Wu and Woo, 1983). This is the first study to document the osmoregulatory capacity of juvenile goliath grouper in low salinity water and freshwater. Inland aquaculture of goliath grouper in brackish or freshwater systems may have far-reaching implications on the local farming landscape, be important to the socioeconomic well-being of communities, while causing minimal impact to the environment.
Notes
¤To cite this article: García LN, Sierra CL, Perez J, Esquivel F, Chapman FA. Osmoregulation of juvenile marine goliath grouper (Epinephelus itajara) in lowsalinity water. Rev Colomb Cienc Pecu 2013; 26:127-135.
Acknowledgments
We wish to thank the fisherman on the scene for their support, Professor V. Atencio from Universidad de Córdoba, and also the Corporación Para el Desarrollo de los Valles del Sinú y San Jorge (CVS) in Puerto Cispatá (Colombia) for allowing us to use their facilities. This study is part of the thesis by L.N. García, to be submitted to the Universidad Católica del Norte in Coquimbo (Chile), in partial fulfillment of the requirements for the degree of Magíster en Acuicultura. This study was funded in part by the University of Florida and Universidad del Pacífico (Colombia).
References
Bancroft JD, Gamble B. Theory and practice of histological techniques. Philadelphia (PA): Churchill Livingstone; 2002. [ Links ]
Botero J, Ospina J F. Crecimiento y desempeño general de juveniles silvestres de mero guasa Epinephelus itajara (Lichtenstein) mantenidos en jaulas flotantes bajo diferentes condiciones de cultivo. Bol Invest Mar Costeras 2003; 32:25- 36. [ Links ]
Bullock LH, Murphy MD, Godcharles MF, Mitchell ME. Age, growth, and reproduction of jewfish Epinephelus itajara in the eastern Gulf of Mexico. Fish Bull 1992; 90:243-249. [ Links ]
Cervigon F. La acuicultura en Venezuela: estado actual y perspectivas. Caracas (Venezuela): Editorial Artes; 1983. [ Links ]
Choe K and Strange K. Volume regulation and osmosensing in animal cells. In: Evans DH, editor. Osmotic and ionic regulation: cells and animals. Boca Raton: CRC Press; 2009. p.37-67. [ Links ]
Eklund AM. Habitat affinities of juvenile goliath grouper to assess estuarine conditions. In: Bortone SA, editor. Estuarine indicators. Boca Raton: CRC Press; 2005. p.393-407. [ Links ]
Evans DH. Osmotic and ionic regulation: cells and animals. Boca Raton (FL): CRC Press; 2009. [ Links ]
Evans DH, Claiborne JB. Osmotic and ionic regulation in fishes. In: Evans DH, editor. Osmotic and ionic regulation: cells and animals. Boca Raton: CRC Press; 2009. p.295-366. [ Links ]
Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 2005; 85:97-177 [ Links ]
FAO. FAO Yearbook of fishery and aquaculture statistics, 2008. Rome: FAO; 2010. [ Links ]
Frias-Torres S. Habitat use of juvenile goliath grouper Epinephelus itajara in the Florida Keys, USA. Endang Species Res 2006; 2:1-6. [ Links ]
Hostim-Silva M. The ''lord of the rock's'' conservation program in Brazil: the need for a new perception of marine fishes. Coral Reefs 2005; 24:74. [ Links ]
IUCN. Epinephelus itajara. The IUCN red list of threatened species. Version 2011.2. International Union for Conservation of Nature and Natural resources, IUCN, 2011 [Access date: November, 2011]. URL: http://www.iucnredlist.org/apps/redlist/details/195409/0 [ Links ]
Kefford BJ, Papas PJ, Metzeling L, Nugegoda D. Do laboratory salinity tolerances of freshwater animals correspond with their field salinity?. Environ Pollut 2004; 129:355-362. [ Links ]
Koenig CC, Coleman FC, Eklund AM, Schull J, Ueland J. Mangroves as essential nursery habitat for goliath grouper (Epinephelus itajara). Bull Mar Sci 2007; 80:567-586. [ Links ]
Mejía LS, Acero A. Libro rojo de peces marinos de Colombia. INVEMAR; 2002. [ Links ]
Moyle PB and Cech JJ. Fishes: an introduction to ichthyology. Englewood Cliffs (NJ): Prentice-Hall; 1982. [ Links ]
NMFS. Status report on the continental United States distinct population segment of the goliath grouper (Epinephelus itajara). National Marine Fisheries Service, NMFS; 2006; [Accessed date: November 2011] URL: http://sero.nmfs.noaa.gov/pr/pdf/Final_Status_Report_on_the_Goliath_Grouper.pdf [ Links ]
Okada T, Yoneshima H, Mukai Y, Sawada Y. Tolerance limits of young kelp grouper Epinephelus moara for environmental stresses. Bull Fish Lab Kiniki Univ 1996; 5:139-146. [ Links ]
Sadovy Y, Eklund AM. Synopsis of biological information on the Nassau grouper, Epinephelus striatus (Bloch 1792) and the jewfish, E. itajara (Lichtenstein 1822). NOAA Tech. Rep. NMFS 146; 1999. [ Links ]
Smith CL. A revision of the American groupers: Epinephelus and allied genera. Bull Am Mus Nat Hist 1971; 146(2):67-242. [ Links ]
Tasker, JB. Fluids, electrolytes, and acid-base balance. In: Kaneko JJ, editor. Clinical biochemistry of domestic animals. Third edition. New York: Academic Pres; 1980. p.401-446. [ Links ]
Timmons MB, Ebeling JM, Wheaton FW, Summerfelt ST, Vinci BJ. Recirculating aquaculture systems. 2nd ed. Ithaca (NY): Cayuga Aqua Ventures; 2002. [ Links ]
Torossi SC. 1982: Aspectos biológicos y ensayo de cultivo del mero guasa Epinephelus itajara (Lichtenstein, 1822) (Pisces: Serranidae) en la laguna de la Restinga (Isla de Margarita- Venezuela). Tesis de Grado, Universidad de Oriente, Venezuela. [ Links ]
Tupper M, Sheriff N. Capture-based aquaculture of groupers. In: Lovatelli A and Holthus PF, editors. Capture-based aquaculture, global overview. FAO Fisheries Technical Paper, no. 508. Rome: FAO; 2008. p.217-253. [ Links ]
Uchida K, Kaneko T. Enhanced chloride cell turnover in the gills of chum salmon fry in seawater. Zool Sci 1996; 13:655- 660. [ Links ]
Woo NYS, Wu RSS. Metabolic and osmoregulatory changes in response to reduced salinities in the red grouper Epinephelus akaara (Temminck & Schlegel), and the black sea bream, Mylio macrocephalus (Basilewsky). J Exp Mar Biol Ecol 1982; 65:139-161. [ Links ]
Wu RSS, Woo NYS. Tolerance of hypo-osmotic salinities in thirteen species of adult marine fish: implications for estuarine fish culture. Aquaculture 1983; 32:175-181. [ Links ]