Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Colombiana de Ciencias Pecuarias
versão impressa ISSN 0120-0690
Rev Colom Cienc Pecua vol.27 no.1 Medellín jan./mar. 2014
ORIGINAL ARTICLES
Application of a polymerase chain reaction test for the detection of Brucella canis from clinical samples of canines and humans¤
Aplicación de una prueba de reacción en cadena de la polimerasa para detección de Brucella canis a partir de muestras clínicas de caninos y humanos
Aplicação de um teste de reacção em cadeia da polimerase para detecção de Brucella canis a partir de amostras clínicas de canino e humano
Miryan M Sánchez-Jiménez1, Bact, Msc; Luisa F Ortiz-Román2, MyB; Laura L Castrillón-Salazar2, MV; Carlos A Giraldo-Echeverri2, MV, Msc; Martha Olivera-Angel1*, MV, Dr, Sci agr.
1Grupo Vericel, Facultad de Ciencias Agrarias, Universidad de Antioquia. Carrera 75 # 65-87. Medellín, Colombia.
2Grupo Biogénesis, Facultad de Ciencias Agrarias, Universidad de Antioquia. Carrera 75 # 65-87, Medellín, Colombia.
* Corresponding autor: Martha Olivera-Angel. Universidad de Antioquia, Facultad de Ciencias Agrarias, Grupo Vericel. Carrera 75 # 65-87. Medellín, Colombia. Email: syngamia@gmail.com
(Received: April 22, 2013; accepted: November 1, 2013)
Summary
Background: laboratory diagnosis of canine brucellosis includes serological and bacteriological tests; the blood culture is considered the gold standard, but it presents issues of sensitivity and delay in results. Therefore, the polymerase chain reaction (PCR) could be useful to detect low amounts of bacterial DNA from clinical samples and provide results within hours. Objective: to evaluate the sensitivity and specificity of PCR for the detection of Brucella canis in whole blood samples. Methods: blood samples from 499 dogs from kennels in two Colombian regions and 91 co-inhabiting humans were used. The 2-mercaptoethanol rapid slide agglutination test (2ME-RSAT) from serum and blood culture and PCR tests from whole blood were performed on all samples. Bayes theorem was used to establish the sensitivity and specificity of the PCR test compared with the other tests performed. Results: 9.9% of the evaluated co-inhabiting humans yielded positive serological results and 0% were positive by PCR or blood culture tests. 10.8% of dog samples were positive by blood culture, 19% were positive by PCR and 13% were positive by 2ME-RSAT. 7% of the samples were positive by all tests. Compared with blood culture, PCR had a sensitivity of 92.6% and a specificity of 90% for canine samples. Compared with 2ME-RSAT, it had a sensitivity of 77.4% and a specificity of 89.2%. When PCR and 2ME-RSAT results were compared with blood culture, a higher number of positive samples were retrieved than when results of only individual tests were applied. Conclusions: PCR is useful to detect B. canis in clinical samples; however, it is preferable to include the 2ME-RSAT test, as this improves the accuracy of the diagnosis. The PCR results are obtained within 24 to 48 hours and do not require the presence of whole bacterial cells to detect DNA.
Key words: canine brucellosis, diagnosis, kennels, zoonoses.
Resumen
Antecedentes: el diagnóstico de laboratorio de brucelosis canina incluye pruebas serológicas y bacteriológicas. El hemocultivo se considera el estándar de oro, pero tiene problemas de sensibilidad y tiempo de entrega de los resultados. Por eso, la reacción en cadena de la polimerasa (PCR) podría ser útil para detectar pequeñas cantidades de ADN bacteriano de muestras clínicas y proporcionar resultados en horas. Objetivo: evaluar la sensibilidad y especificidad de una PCR para la detección de Brucella canis, en muestras de sangre total de 499 perros de perreras y 91 humanos cohabitantes de dos regiones de Colombia. Métodos: se realizaron pruebas de aglutinación rápida en placa con 2-mercaptoetanol (2ME-PARP) a partir de suero, PCR y hemocultivo a partir de sangre total. El teorema de Bayes se utilizó para establecer la sensibilidad y especificidad de la prueba de PCR respecto a las demás. Resultados: 9,9% de los humanos cohabitantes evaluados resultaron positivos para la prueba serológica, y el 0% fue positivo para PCR o hemocultivo. 10,8% de las muestras de perros fueron positivas para hemocultivo, 19% fueron positivas para PCR y 13% eran 2ME-PARP positivo. 7% de las muestras fueron positivas para todos los ensayos. En las muestras caninas, la PCR tuvo una sensibilidad del 92,6% y una especificidad del 90% en comparación con el hemocultivo. En comparación con 2ME-PARP, tuvo una sensibilidad del 77,4% y una especificidad del 89,2%. Cuando se compararon los resultados de la PCR y 2ME-PARP con el hemocultivo, un mayor número de muestras positivas fueron obtenidas que usando los resultados de cada una. Conclusiones: la PCR es útil para detectar B.canis en muestras clínicas, sin embargo, es recomendable incluir la prueba de 2ME-PARP, lo que mejora la exactitud del diagnóstico, se obtienen los resultados en 24 ó 48 horas y no requieren la presencia de células bacterianas completas para detectar ADN.
Palabras clave: brucelosis canina, diagnóstico, criaderos, zoonosis.
Resumo
Antecedentes: o diagnóstico laboratorial da brucelose canina inclui testes sorológicos e bacteriológicos. O padrão ouro é a cultura de sangue, mas tem problemas com a sensibilidade e o tempo de entrega dos resultados. Por conseguinte, a reação em cadeia da polimerase (PCR), pode ser útil para detectar pequenas quantidades de ADN bacteriano diretamente a partir de amostras clínicas e fornecem resultados em 24 horas. Objetivo: para avaliar a sensibilidade e a especificidade da PCR para a detecção de Brucella canis em amostras de sangue total de 499 caninos provenientes de canis e 91 seres humanos em contato com estes cães em duas regiões da Colômbia. Métodos: foram realizados testes de aglutinação rápida em placa com 2-mercaptoetanol (2ME-PARP) a partir de soro, PCR y hemocultura a partir de sangue total. O teorema de Bayes utilizou-se para estabelecer a sensibilidade e especificidade da PCR em relação aos outros. Resultados: 9,9% (9) dos humanos avaliados resultaram positivos na prova sorológica, 100% foram negativos por PCR e hemocultura. 10.8% (54) das amostras de cães foram positivas na hemocultura, 19% (95) foram positivas na PCR e 13% (65) foram positivas no teste 2ME-PARP. 7% (35) das amostras foram positivas para todos os testes. Nas amostras caninas, a PCR teve sensibilidade do 92.6% e uma especificidade de 90% em comparação com a hemocultura. Em comparação com 2ME-PARP, teve uma sensibilidade de 77.4% e uma especificidade de 82.2%. Quando foram comparados os resultados da PCR e 2-ME-PARP com a hemocultura, um maior número de amostras positivas foram obtidas que quando foram usados os resultados de cada uma. Conclusões: a PCR é útil para detectar B. canis em amostras clínicas, porém, é recomendável incluir o teste de 2ME-PARP, para melhorar a acurácia do diagnóstico. Os resultados obtém-se em 24-48 horas e não requerem a presença de células bacterianas completas para detectar ADN.
Palavras chave: Brucelose canina, canil, diagnóstico, zoonoses.
Introduction
Canine brucellosis is caused by Brucella canis, an intracellular, rough and small Gram-negative bacterium (Ebani et al., 2010) that is widespread in America, Europe, and Asia (Giraldo et al., 2009; Gyuranecz et al., 2011; Kang et al., 2011) and causes abortions and reproductive failure in dogs (Jones et al., 1968). In canines, the bacterium is present in placental materials, aborted fetuses, vaginal discharge of infected dogs, milk (Olivera et al., 2011), urine, and seminal and prostate fluids (Carmichael et al., 1990) that are contagious to other dogs and humans. Non-specific symptoms of the disease include lethargy, loss of libido, uveitis, premature aging and generalized lymph node enlargement (Wanke, 2004).
Several cases of human infection have been reported in the past ten years, especially in Latin America; however, pathogenesis of this disease in Latin America differs from that of the rest of the world, probably due to a different B. canis strain (De la Cuesta et al., 2013; Ortiz et al., 2012; Brower et al., 2013). The current number of cases is unknown because cases are rarely diagnosed or reported, and the casuistry of the zoonosis is unknown (Olivera et al., 2009; Lucero et al., 2010; Nomura et al., 2010). Symptoms are usually imprecise, with the presence of persistent fever, enlarged lymph nodes, and other clinical manifestations (Lucero et al., 2005).
Diagnosis can be performed by various serological, bacteriological, and molecular tests. The gold standard for the diagnosis of B. canis is bacterial isolation from blood cultures, but the animal must be bacteremic to obtain a positive blood culture. The sensitivity of this test ranges from 20 to 90%, depending on the stage of the disease (Keid et al., 2009).
Serological tests are useful to detect the presence of antibodies in serum; the 2-mercaptoethanol rapid slide agglutination test (2ME-RSAT), Indirect Immunofluorescence, and ELISA have been used with variable results (Carmichael et al., 1987; Ebani et al., 2003; Lucero et al., 2002). Surface antigens of rough Brucella species can cross react with antibodies produced against other pathogenic species of bacteria, causing variations in the sensitivity and specificity of these tests (Noosud et al., 2009; Escobar et al., 2010). Because of this it is necessary to use blood culture to confirm B. canis infection. However, at times, this bacteria cannot be isolated due to low levels of colony-forming units circulating in the blood. This could be caused by a focalization of the infectionâas in chronic casesâ or due to the use of antibiotics for other causes, which decrease bacteria circulating in the blood.
Several molecular biology tests such as the Polymerase chain reaction (PCR) have been recently developed for the direct detection of B. canis in different types of clinical samples (Aras et al., 2010; Imaoka et al., 2010; Olivera et al., 2011). The sensitivity and specificity of these tests vary. These are convenient tools for detecting bacterial DNA and merely require the presence of genetic material.
These considerations demonstrate the need for a rapid, sensitive, and specific method to diagnose infection in dogs and humans; a method that could be used along with blood culture, because blood cultureâalthough it is the gold standardâis time consuming and is not widely available. This could facilitate the diagnosis and reduce its time, thus reducing the complications associated with the disease. We report the application of PCR using primers designed to identify Brucella species (Garcia-Yoldi et al., 2006) directly from the whole blood of canine and co-inhabiting humans to increase speed, sensitivity, and specificity of the report.
Materials and methods
Sampling
The study included 499 dogs from 21 kennels located in two regions of Antioquia (Colombia), that entered the study consecutively, and whose owners voluntarily agreed to participate in the project by signing an informed consent. The distribution of kennels, canines, and humans was as follows: Region 1 (Urban Area): 11 kennels, 300 canines, and 52 humans; Region 2 (Rural area): 10 kennels, 199 canines, and 39 humans.
Sampling was conducted in each of the 21 kennels. Canine blood samples were taken in Vacutainer tubes either dry or with 3.2% citrate added (Becton Dickinson, Franklin Lakes, NJ, USA) to obtain serum or whole blood, respectively. Three milliliters of blood was inoculated into the bottle for blood culture. The same procedure was conducted in each human participant.
Laboratory Tests
2ME-RSAT was performed on all samples as follows: 25 μL of blood serum were mixed with 25 μL 2-mercaptoethanol followed by 50 μL B. canis total antigen produced from the M-strain (a less mucoid B. canis strain used as antigen for serological diagnosis of canine brucellosis), mixed with a stick and kept in an orbital shaker for two minutes. An agglutination viewer was used to determine presence of agglutination. The test is positive when agglutination is observed (Carmichael et al., 1996).
Blood culture was conducted as follows: 5 mL human blood or 3 mL canine blood where inoculated in tripticase soy broth medium (Becton Dickinson, Franklin Lakes, NJ, USA) and incubated for 2 days at 37 °C. On alternating days during the first week, a sample from the blood culture was seeded in tripticase soy agar and incubated at 37 °C (Keid et al., 2009). If Brucella-compatible growth was detected, Gram tests and biochemical identification tests, namely urease production in urea-supplemented medium and sulphur indole motility test, were conducted according to Lucero et al. (2008). In the event that no Brucella-compatible growth was detected during the first week, blood cultures were maintained at 37 °C and inoculated in tripticase soy agar every week for 2 months before being conclusively considered as negative.
DNA extraction
DNA isolation was prepared from canine and human blood samples using the dneasy blood and tissue kit (QIAGEN Inc., Valencia, CA, USA), following manufacturer's instructions for DNA extraction from whole blood. Each DNA sample was diluted 1:100 and used immediately or stored at -20 °C until needed.
PCR primers
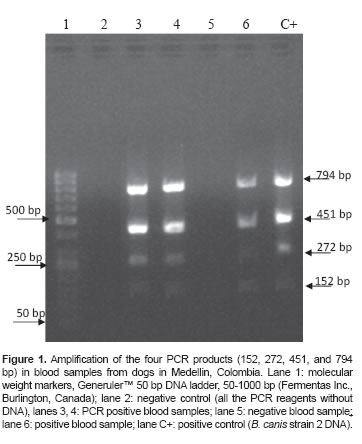
The PCR primers used were designed by Garcia- Yoldi et al. (2006). These primers amplify three bands: a 152 bp band positive for all Brucella species except for B. neotomae; a 272 bp band present only in B. canis, B. suis, and B. neotomae; and a 794 bp band positive in B. canis group 2. An additional primer pair that amplifies a Brucella genus-specific 451 bp band present in all Brucella species was also used (Table 1). Despite not having been reported as B. canis-specific by Garcia- Yoldi et al. (2006), the 794 bp band was detected and determined as such in a previous study on Colombian strains belonging to B. canis Group 2 (Ortiz et al., 2012). The combination of primers listed in table 1 was used to detect at least one of these fragments in each sample, in order to be considered positive for B. canis infection.
PCR conditions
The PCRs were performed as previously reported by Ortiz et al. (2012). Each sample was run in triplicate. A total volume of 25 μL was used, containing 3 μL of 1 ng/μL DNA template, 0.625 μL of 0.25 μM of each primer (Table 1), 0.2 μL of Taq DNA polymerase (5 IU/μL, Fermentas Taq DNA polymerase recombinant. Foster City, CA, USA), 3 μL of 3 mM MgCl2 (Fermentas, Foster City, CA, USA), 2.5 μL of 10X buffer with Tris-HCl, Triton X-100, and KCl (pH 8.8) (Fermentas, Foster City, CA, USA), and 0.5 μL of 10 mM of each dNTP, and completed with distilled water. Amplification was run in a PTC 200 thermocycler (Perkin-Elmer Inc., San Jose, CA, USA) for 25 cycles, as reported by García- Yoldi et al. (2006). Water was added to the negative control. DNA from the attenuated Carmichael B. canis M- strain, a less mucoid strain used to prepare antigen for the serologic test, served as positive control (Carmichael and Joubert, 1987).
Filter pipette tips and pipettes for PCR were used exclusively to avoid contamination with DNA carry over. Protocol of disinfection and cleaning of pipettes and surfaces before and after PCR procedure were also applied.
Detection of PCR products
The PCR products were analyzed in 1% agarose gels containing 0.5 μg/ml ethidium bromide, and detected under UV light using a photo-gel imaging system (Transilluminator Mini Benchtop Model M-10E, UVP, Upland, CA, USA)
Limit of detection of the PCR product
To establish the lowest amount of DNA detectable by this PCR, DNA from the B. canis M-strain was extracted and quantified. A hundredfold serial dilution of the DNA sample was carried out using distilled water. Such dilutions were used as templates for PCR. The detection limit was determined by gel electrophoresis, defined as the last dilution where bands were detected.
Results analysis using two-by-two contingency tables
The results of the different tests were compared as follows: PCR vs. 2ME-RSAT, PCR vs. blood culture, and 2ME-RSAT vs. blood culture. The sensitivity (S), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) were determined using two-by-two contingency tables.
Ethical statement
This study was conducted based on ethical approval for animal (law 84/1989 Colombia's National Statute for the Protection of Animals) and human (Resolution No. 008430/1993 Ministry of Health of Colombia) experiments. Samples were taken after participating humans and dog owners signed an informed consent form. This work was conducted under the highest standards of animal care.
Results
The Multiplex PCR test does not yield ideal results when DNA is extracted directly from whole blood due to an uneven amplification pattern. The signal of the 152 and 272 bp bands is usually weak, whereas the 451 and 794 bp bands yield a better signal (Figure 1).
The limit of detection of the PCR -the most diluted sample in which all four bands were visible in the gelwas determined to be 0.38 pg DNA (Figure 2).
When regions were compared, it was found that 10 of the 11 kennels in the urban area were positive by at least one diagnostic test, while in rural areas 9 of the 10 kennels were negative by all tests. The most prevalent symptoms in infected kennels were related to reproductive failures, such as abortion and infertility. There were no reports of B. canisinfection symptoms in negative kennels.
A total of 54 dogs (10.8%) were blood-culture positive, 95 (19%) were PCR positive, 62 (12.4%) presented a positive serology, and 35 (7%) were positive by all tests.
For all tests combined, the results were: 0.4% of the samples were 2ME-RSAT, blood culture positive and PCR negative; 3% of the samples were blood culture, PCR positive and 2ME-RSAT negative; 2.6% of the samples were 2ME-RSAT and PCR positive but blood culture negative and 2.4% were only 2ME-PARP positive; 0.4 and 6.4% of the samples were only positive to blood culture and PCR, respectively; and 77.8% were negative by all three tests.
Of the 91 humans studied, 9 (9.9%) presented a positive serology, all corresponding to the urban area, and 100% in both regions were negative for B. canis by both PCR and blood culture.
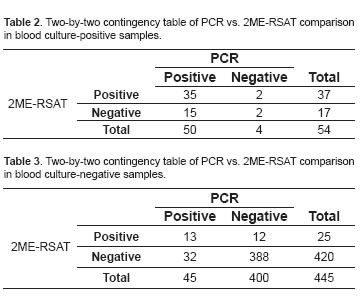
The contingency tables constructed for the comparison of the different tests in dogs are shown in tables 2 to 4.
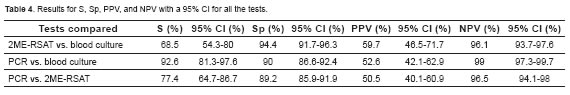
Table 4 describes results for S, Sp, PPV, NPV with minimum and maximum values and a 95% confidence interval (CI) when all three tests were compared.
Discussion
Our results were obtained using primers previously reported to identify Brucella species, but that had problems because B. canis and B. suis were not discriminated. Recently Lopez-Goñi et al. (2011) and Kang et al. (2011) proposed the use of different sets of primers to solve this problem in different strains. Our strategy was based on the use of primers previously reported by Garcia Yoldi et al. (2006) and, in the present study they are used directly in clinical samples and not in strains. The primers selected were previously used (Ortiz et al., 2011) to classify strain isolates in dogs in Medellin, Colombia. In the previously conducted in silico analysis, unspecificities in humans or dogs were not detected. The primers were chosen because the circulating B. canis was classified as Group 2, so that amplification of this band in clinical samples was an indicator of B. canis infection.
Blood culture is the gold standard and unequivocal test to confirm infection by B. canis, but diagnostic tests should have high sensitivity and specificity, and also be quick in delivering results. The combination of these features allows a test to be useful for diagnosing a disease. PCR test evaluated in the present study had a 92.6% sensitivity and 90% specificity when compared with blood culture. This could be due to the PCR detecting circulating bacterial DNA, while the blood culture requires the microorganism to be intact and viable in order to be isolated. Another reason for PCR positive but blood culture negative samples could be that owners did not report canines that were receiving antibiotics.
Sensitivity of our PCR was similar to that reported by Keid et al. (2010) who tested PCR directly from canine whole blood (n = 72) and found 97.14% sensitivity. With regard to specificity, Keid et al. (2010) reported 100% specificity, whereas our results are somewhat lower. This difference may be explained by the fact that our study was undertaken on a larger number of dogs and was a multiplex PCR assay, while the work of Keid et al. (2010) was based on PCR with one gene as single target, which could have influenced the results.
The results reported here also differ from a study by Noosud et al. (2009), who reported 100% sensitivity and specificity for a PCR test that amplifies the 16S rRNA gene of B. canis when compared with blood culture, while PPV was 52.6% and NPV was 99%. When PCR was compared with 2ME-RSAT serological test, it had 77.4% sensitivity and 89.2% specificity. This might occur because PCR is considered a direct test whereas 2ME-PARP is an indirect test. PCR test can be positive in acute cases with recent bacteremia and still no production of antibodies, causing the serological test to be negative. The opposite can also occur: PCR can be negative in cases of chronic infection because of the lack of bacteria circulation and localization in organs such as the mesenteric lymph nodes. In such cases, serology may be positive because antibodies were previously produced, as reported by Keid et al. (2009). As for PCR false negatives, another reason may be the presence of inhibitors in the blood, such as hemoglobin and immunoglobulin G, as reported by Queipo Ortuño et al. (2008).
The 13 animals with PCR and 2ME-RSAT positive but blood culture negative belong to kennels with dogs positive to infection, so they could be considered positive dogs. 44 dogs with 2ME-RSAT or positive PCR outcome but negative blood culture results are considered suspects; in ideal conditions a follow up should be conducted (taking new samples should be considered).
The presence of PCR or blood culture negative but serologically positive humans may be explained by a previous infection with antibody development and bacteria absence in blood. Another reason was proposed by Zerva et al. (2001), who concluded that the best sample in humans is blood serum and not whole blood, precisely because of the large amount of inhibitors blood may contain.
Olivera et al. (2011) reported the use of the same set of primers as the one used here and applied them to DNA extracted from milk and whole blood from a dog and the puppy. In that previous study samples yielded more positive results by PCR tests than by blood culture tests, supporting the proposal that PCR only requires the presence of DNA and not metabolically active bacteria.
The utility of a multiplex PCR test is that it allows simultaneous amplification of several target genes. However, it is seldom used in clinical samples, as it was designed for bacterial isolates. Based on the results presented here, while this test is appropriate for use in clinical samples, not all of the evaluated genes are good targets for detection. We conclude that PCR is useful for B. canis detection in clinical samples, but it is better when 2ME-RSAT is also used, because this improves sensitivity and specificity, the results are rapid (being obtained within 24 to 48 hours), and the presence of whole bacterial cells is not required to detect DNA, as it is the case with blood culture. In conclusion, 2MERSAT is mainly used to diagnose canine brucellosis, while PCR and isolation of the infectious agent are also used as complementary methods for early or acute infection. The combined use of these tests could improve accuracy of diagnosis.
¤ To cite this article: Sánchez-Jiménez MM, Ortiz-Román LF, Castrillón-Salazar LL, Giraldo-Echeverri CA, Olivera-Angel M. Application of a polymerase chain reaction test for the detection of Brucella canis from clinical samples of canines and humans. Rev Colomb Cienc Pecu 2014; 27:3-11.
Acknowledgments
This work was supported by Convocatoria Regionalización 2010- CODI Universidad de Antioquia to Vericel Research Group, Universidad de Antioquia sustainability strategy 2013-2014, and by COLCIENCIAS through a contractual research grant for PhD students and Young Scientists.
We also thank biologist Juan Jacobo de la Cuesta for his help in the translation of this paper and Anne-Lise Haenni for English proofreading.
References
Aras Z, Ucab US. Detection of Brucella canis from inguinal lymph nodes of naturally infected dogs by PCR. Theriogenology 2010; 74:658-462. [ Links ]
Banai M, Corbel M. Taxonomy of Brucella. Open Vet Sci J 2010; 4:85-101. [ Links ]
Brower A, Lucero N, Okwumabua O, Gopaul KK, Whatmore AM, Cravero SL, Trangoni MD. Newly Identified Variability in Brucella canis Fatty-Acid Content Is Associated With Geographical Origin. Epidemiol Infect 2013; 141:852-858. [ Links ]
Carmichael LE, Joubert JC. A rapid slide agglutination test for the serodiagnosis of Brucella canis infection that employs a variant (M-) organism as antigen. Cornell veterinary 1987; 77:3-12. [ Links ]
Carmichael LE, Green EG. Canine brucellosis. In: Greene CE, editor. Infectious diseases of the dog and cat. Philadelphia: W.B. Saunders; 1990. [ Links ]
Carmichael LE, Shin SJ. Canine brucellosis: a diagnostician's dilemma. Semin Vet Med Surg 1996; 11:161â165. [ Links ]
De la Cuesta-Zuluaga JJ, Sánchez-Jiménez MM, Martinez- Garro J, Olivera-Angel M. Identification of the virB operon genes encoding the type IV secretion system, in Colombian Brucella canis isolates. Vet Microbiol 2013; 163:196â199. [ Links ]
Díaz R, Casanova A, Ariza J, Moriyón I. The rose bengal test in human brucellosis: a neglected test for the diagnosis of a neglected disease. PLoS Negl Trop Dis 2011; 5:e950. [ Links ]
Ebani VV, Cerri D, Fratini F, Bey RF, Andreani E. Serological diagnosis of brucellosis caused by Brucella canis. New Microbiol 2003; 26:65-73. [ Links ]
Escobar GI, Boeri EJ, Ayala SM, Lucero NE. The feasibility of using antigens prepared with rough Brucella strains for diagnosis of canine brucellosis. Rev Arg Microbiol 2010; 42:35- 40. [ Links ]
García-Yoldi D, Marín CM, de Miguel MJ, Muñoz PM, Vizmanos JL, López-Goñi I. Multiplex PCR Assay for the identification and differentiation of all Brucella species and the vaccine strains Brucella abortus S19 and RB51 and Brucella melitensis Rev1. Clin Chem 2006; 52:779-781. [ Links ]
Giraldo C, Ruiz-Cortés T, Olivera M. Brucella canis in Medellín (Colombia), a current problem. Rev Udca Actual Divulg Cient 2009; 12:51-57. [ Links ]
Gyuranecz M, Szeredi L, Ronai Z, Denes B, Dencso L, Dan A, Palmai N, Hauser Z, Lami E, Makrai L. Detection of Brucella canis-induced reproductive diseases in a kennel. J Vet Diagn Invest 2011; 23:143-147. [ Links ]
Imaoka K, Kimura M, Suzuki M, Kamiyama T, Yamada A. Simultaneous detection of the genus Brucella by combinatorial PCR. Jpn J Infect Dis 2007; 60:137â139. [ Links ]
Jones LM, Zanardi M, Leong D, Wilson JB. Taxonomic position in the genus Brucella of the causative agent of canine abortion. J Bacteriol 1968; 95:625-630. [ Links ]
Kang SI, Heo EJ, Cho D, Kim JW, Kim JY, Jung SC, Her M. Genetic comparison of Brucella canis isolates by the MLVA assay in South Korea. J Vet Med Sci 2011; 73:779-786. [ Links ]
Kang SI, Her M, Kim JW, Kim JY, Ko KY, Ha YM, Jung SC. Advanced multiplex PCR assay for differentiation of Brucella species. Appl Environ Microbiol 2011; 77:6726-6728. [ Links ]
Keid LB, Soares RM, Vasconcellos SA, Megidb J, Salgado VR, Richtzenhain LJ. Comparison of agar gel immunodiffusion test, rapid slide agglutination test, microbiological culture and PCR for the diagnosis of canine brucellosis. Res Vet Sci 2009; 86:22- 26. [ Links ]
Keid LB, Soares RM, Vasconcellos SA, Salgado VR, Megidb J, Richtzenhain LJ. Comparison of a PCR assay in whole blood and serum specimens for canine brucellosis diagnosis. Vet Rec 2010; 167:96-99. [ Links ]
López-Goñi I, García-Yoldi D, Marín CM, De Miguel MJ, Barquero-Calvo E, Guzmán-Verri C, Albert D, Garin-Bastuji B. New Bruce-ladder multiplex PCR assay for the biovar typing of Brucella suis and the discrimination of Brucella suis and Brucella canis. Vet Microbiol 2011; 154:152-155. [ Links ]
Lucero NE, Escobar GI, Ayala SM, Lopez G. Sensitivity and specificity of an indirect enzyme-linked immunoassay for the diagnosis of Brucella canis infection in dogs. J Med Microbiol 2002; 51:656-660. [ Links ]
Lucero NE, Escobar GI, Ayala SM, Jacob N. Diagnosis of human brucellosis caused by Brucella canis. J Med Microbiol 2005; 54:457-461. [ Links ]
Lucero NE, Corazza R, Almuzara MN, Reynes E, Escobar GI, Boeri E, Ayala SM. Human Brucella canis outbreak linked to infection in dogs. Epidemiol Infect 2010; 138:280-285. [ Links ]
Lucero NE, Escobar GI, Ayala SM, Hasan DB. Manual de Procedimientos. Para el Diagnóstico de brucelosis humana. 2008 OPS; [Access date: April 22 2013]. URL: http://www.panalimentos.org/salmsurv/file/manuales/Manual%20de%20Procedimientos%20Brucelosis_2008.pdf. [ Links ]
Nomura A, Imaoka K, Imanishi H, Shimizu H, Nagura F, Maeda K, Tomino T, Fujita Y, Kimura M, Stein G. Human Brucella canis infections diagnosed by blood culture. Emer Infect Dis 2010; 16:1183-1184. [ Links ]
Noosud J, Sirinarumitr K, Sirinarumitr T. Comparison of a serological method, a bacteriological method and 16rRNA Brucella canis PCR for canine brucellosis diagnosis. Kasetsart J (Nat Sci) 2009; 43:159-164. [ Links ]
Olivera M, Di Lorenzo C. Aislamiento de Brucella canis en un humano conviviente con caninos infectados. Informe de un caso. Colombia Médica 2009; 40:218- 220. [ Links ]
Olivera M, Giraldo CA, Di-Lorenzo C. PCR Identification of Brucella canis in canine blood and milk. A case report. Arch Med Vet 2011; 43:295-298. [ Links ]
Ortíz LF, Muskus C, Sánchez MM, Olivera M. Identification of Brucella canis Group 2 in colombian kennels. Rev Colomb Cienc Pecu 2012; 25:615-619. [ Links ]
Queipo-Ortuño MI, Colmenero JD, Macias M, Bravo JM, Morata P. Preparation of Bacterial DNA Template by Boiling and Effect of Immunoglobulin G as an Inhibitor in Real-Time PCR for Serum Samples from Patients with Brucellosis. Clin Vaccine Immunol 2008; 15:293-296. [ Links ]
Wanke MM. Canine brucellosis. Animal Repr Sci 2004; 82- 83:195- 207. [ Links ]
Zerva L, Bourantas K, Mitka S, Kansouzidou A, Legakis NJ. Serum is the preferred clinical specimen for diagnosis of human brucellosis by PCR. J Clin Microbiol 2001; 39:1661-1664. [ Links ]