Introduction
Synchronization has been widely used to program lambing, increasing the number of lambs, and to introduce semen of high genetic merit into the herd (Cleef et al., 1998; Godfrey et al., 1999). Progesterone is the most widely used hormone in these controlled breeding protocols (Fleisch et al., 2006; Hashemi et al., 2006; Seekallu et al., 2010). Several researchers have reported that maternal recognition and implantation, during the onset of gestation, is when most fertility failures occur (Fthenakis et al., 2012).
Using exogenous progesterone during synchronization increases lipid oxidation (Sönmez et al., 2009). Moreover, it has been reported that metabolic changes modify the oxidative state of the animals, possibly causing oxidative stress (See-Ling et al., 2013). According to Al-Gubory et al. (2010), this stress can cause failures in reproductive processes, such as damage to lipids, proteins and DNA, infertility and miscarriages. Knowledge of the oxidative status before and after estrus synchronization may help determining when to supplement antioxidants. It has been reported that antioxidants such as vitamin E before insemination increases prolificacy and reduces lipid oxidation (Sönmez et al., 2009).
Coffee pulp is a byproduct freely available in coffee growing regions. It has high contents of phenolic compounds and has strong antioxidant properties (Arellano et al., 2011). However, it also contains 0.87% caffeine (Ferreira et al., 2001), which is not recommended in humans, since it delays fetal development during the last third of pregnancy (Vik et al., 2003) and causes embry loses in early pregnancy (Stefanidou et al., 2011). Some byproducts of coffee processing have been tested in dairy cows. Findings show that up to 150 g/Kg coffee pulp can be included in cow diets without affecting production (Cipriano et al., 2006), or 25% in the concentrate at a 60:40 forage: concentrate ratio (Soares et al., 2007a; Soares et al., 2007b). Although inclusion of coffee byproducts in animal diets has been acceptable up to given inclusion levels, it is necessary to determine its influence on reproductive variables; on one hand, natural antioxidants help to counteract lipid peroxidation, but on the other, there is controversy over the effects of caffeine in reproduction. So, we hypothesize that changes in the oxidative state during pregnancy and lactation can be modified by 25% inclusion of coffee pulp; however this could affect reproduction. Therefore, the objective of this study was to evaluate the effect of diet supplementation with coffee pulp for 50 days as a nutritional flushing on the antioxidant capacity, lipid oxidation and reproductive characteristics of ewes during estrous synchronization and early gestation.
Material and methods
Ethical considerations
This study was conducted observing the standards for ethics, biosafety and animal well-being of the Colegio de Postgraduados, Campus Montecillo, Mexico, according to the Official Mexican Standard NOM-033-ZOO-1995 (SAGARPA, 2015).
Experimental design and animal management
The trial was conducted from September (Autumn, reproductive season) to April (Spring, anestrus season) 2014 at the experimental farm of the Colegio de Postgraduados, Campus Montecillo. The climate is temperate subhumid C (Wo) (w)b(i)g with summer rains and 750 mm rainfall (Garcia, 1981). Forty Dorset-Suffolk crossbred ewes with 3 or 4 parturitions were fed oat hay (crude protein: 8.5) and distributed in a completely randomized design to the following two treatments: T0 (n = 21): ewes supplemented with 450 g feed concentrate without coffee pulp (control), and T1 (n = 19): ewes supplemented with 450 g of feed with 25% coffee pulp. The 25% coffee pulp inclusion level was in accordance with previous work conducted in Brazil (Soares et al., 2007a; Soares et al., 2007b).
Supplementation was individually done at 8:00 a.m. The chemical composition of coffee pulp was: caffeine (0.572%), crude protein (10%), ash (6.06), neutral detergent fiber (50.5), and acid detergent fiber (50.2). Ewes were confined in individual pens from 8:00 to 10:00 a.m and later regrouped with free access to oat hay. Concentrate contained 13% crude protein and 2.8 Mcal/Kg dry matter (Table 1), which was adjusted according to the NRC requirements (1985). Ewes were supplemented from the beginning of the experiment when pre-synchronization began (14 days before insertion of progestagen) to 25 days after mating (Figure 1).
Supplementation was aimed to evaluate the effect of coffee pulp, as a nutritional flushing, for up to 25 days. Pregnancy diagnosis was performed 30 and 60 days after CIDR (controlled internal drug release) removal.
Estrous synchronization
At the beginning of the experiment, all ewes were pre-synchronized with two doses of 125 µg prostaglandin (Cloprostenol), 8 days apart. All ewes exhibited estrus after the second administration of prostaglandin, and therefore, it was assumed that they had corpus lutea, and only those that were cycling were used. Six days after the second prostaglandin administration, an intravaginal device impregnated with 0.3 g progesterone (CIDR) was inserted and left for 11 days. Eighteen hours after removing the CIDR, estrous detection initiated with the aid of rams equipped with a protective apron (to prevent mating), and continued at 8-hour intervals (Figure 1). Mating was performed at 8 and 16 h after estrous detection using 8 rams of the same breed with proven fertility. Estrous onset was recorded when the ewes allowed the rams to mount.
Table 1 Diet fed to ewes during estrous synchronization and early pregnancy.

*Phosphorus (17.5%), Sodium (12.9%), Calcium (5.6%), Magnesium (3.4%). T0: Control diet; T1: concentrate with 25% coffee pulp; DM: dry matter.

Figure 1 Synchronization and sampling protocols for measuring the oxidative state and circulating progesterone levels in control ewes and those receiving 25% coffee pulp in the feed concentrate.
Pregnancy diagnosis
Gestation was diagnosed with the aid of real time - Sonovet 600 ultrasound equipment, and using a 7.5 Mhz transducer (Medison, Cypress, California, USA) on days 30 and 60 after removal of CIDR. To calculate the pregnancy rate, the number of ewes diagnosed as pregnant was divided by the number of ewes bred. Prolificacy or fecundity was expressed by dividing the number of lambs born by the number of ewes that lambed. Lamb weight at birth was also recorded during the first 12 hour after parturition.
Assessment of oxidative status
Plasma was obtained to measure oxidative status. Blood samples were collected from the jugular vein in 5 mL tubes with EDTA (ethylenediaminetetraacetic acid) at the beginning of the experiment (before supplementation), before CIDR insertion, and every three days up to day 26 (the day after removing the CIDR devices). After this, samples were collected every 6 days, up to day 50 of the experiment, when concentrate supplementation ended (Figure 1). Tubes were centrifuged at 2500 gravities for 10 min at 4 °C, and plasma was aspirated and stored at -40 °C until analysis.
Total antioxidant capacity was measured with the ferric reducing antioxidant power (FRAP) technique by Benzie and Strain (1996). The 6-hydroxy-2-5-7-8- tetramethyl-chroman-2-carboxylic acid (trolox) was used as a standard at different concentrations (0.2-1.6 nmol/µL) to measure the results.
Lipid oxidation was measured by the thiobarituric acid reactive substances (TBARS) test, according to the technique by Ohkawa et al. (1979). The results were calculated using different concentrations (1-8 nmol) of malonidaldhyde (MDA), recording acid hydrolysis from 1,1,3,3- tetraethoxypropane.
Progesterone measurement
Blood samples were collected from the jugular vein with vacutainer vacuum tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, New Jersey, USA) to measure plasma progesterone (P4). Samples were taken before inserting the CIDR’s and every other day until day 44 of the experiment -which corresponded to one estrous cycle- after removing the CIDR (Figure 1). Plasma P4 was analyzed using the immunoenzyme assay (Immunometrics UK Ltd, 280 London, United Kingdom). The analytical sensitivity was 0.13 ng mL-1, with 9.59 and 13.7% intra and interassay coefficient of variation, respectively.
Statistical analysis
An analysis of variance was conducted for antioxidant capacity and lipid oxidation, with treatment and sampling time as fixed effects. The statistical model used was:
Y ijk =μ +T i + M j + T i M j + Ak (i) + E ijk
Where:
Y ijk = response to the ith treatment in the jth sampling of the kth replication.
μ = general mean.
T i = effect of the ith parturition.
M j = effect of the jth sampling.
T iMj = effect of the ith treatment in the jth sampling.
A k(i) = effect of the ith treatment nested to the kth animal.
E ijk = experimental error.
A chi-squared test was used for fertility analysis and onset of estrus. The GENMOD of SAS was used for prolificacy. Statistical analyses were done using the SAS (2002) Version 9 (SAS Institute Inc, Cary, NC, USA).
Results
Reproductive variables
All ewes (100%) of both treatment groups exhibited estrus after removal of the intravaginal progestogen. Inclusion of 25% coffee pulp in the concentrate did not modify the onset of estrus after progestogen removal (31.61 ± 1.60 h for the control and 34.94 ± 2.61 h for the coffee pulp treatment). The pregnancy rate decreased (p<0.05) by 21.96% at 30 days as an effect of including coffee pulp in the concentrate. However, no pregnancy loss was recorded after this time (the ewes diagnosed pregnant at 30 days were the same animals diagnosed pregnant at 60 days; Table 2).
Table 2 Reproductive variables of ewes supplemented with 250 g coffee pulp/Kg concentrate.
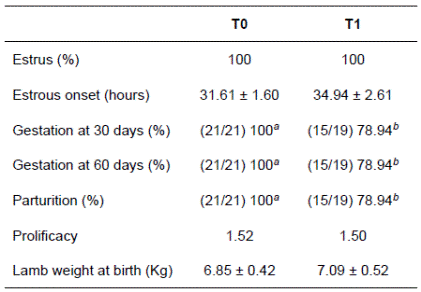
T0: ewes fed 450 g concentrate, (control diet); T1: ewes fed 450 g concentrate with 25% coffee pulp. Different superscript letters (a,b) within the same row indicate statistically significant differences (p<0.05).
Oxidative status
An interaction effect was observed between blood sampling time and treatment on antioxidant capacity. Antioxidant capacity was higher in ewes supplemented with 250 g coffee pulp/Kg than in control ewes at the sampling prior to progestogen insertion. No differences were found between treatments (p<0.05) on the other sampling days. The antioxidant capacity started to decrease from progestogen removal until 22 days into pregnancy in both treatments.
Table 3 Mean (± SE) antioxidant capacity of ewes during estrous synchronization using progestogen and early gestation.
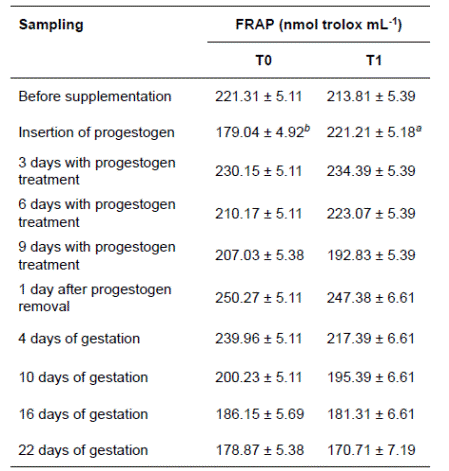
T0: ewes fed 450 g concentrate (control diet); T1: ewes fed 450 g concentrate with 25% coffee pulp. FRAP: ferric reducing antioxidant power.
Lipid peroxidation was not modified by treatment (p>0.05), but there was a change resulting from the sampling time (p<0.05). No changes were recorded before supplementation or during synchronization. However, one day after removing progestogen, when ewes began showing estrus, an increase in lipid oxidation was observed relative to that at the onset of the experiment (when ewes were not pregnant). Nevertheless, lipid oxidation started to decrease after the beginning of gestation, until it reached values below those at 16 and 22 days of pregnancy (Figure 2).

Figure 2 Lipid peroxidation of ewes during estrous synchronization using progestogen during early gestation. Sampling: 1 (before supplementation); 2 (progestogen insertion); 3 (3 days with progestogen treatment); 4 (6 days with progestogen treatment); 5 (9 days with progestogen treatment); 6 (1 day after progestogen removal); 7 (4 days of gestation); 8 (10 days of gestation); 9 (16 days of gestation); 10 (22 days of gestation).T0: ewes fed 450 g concentrate (control diet); T1: ewes fed 450 g concentrate with 25% coffee pulp.
Progesterone
Supplementation with 25% coffee pulp in the concentrate did not modify (p<0.05) plasma progesterone at any sampling time (Table 4). Plasma progesterone increased after progestogen insertion in both treatments, but it decreased in the sampling conducted the day after CIDR removal. Later on, as gestation advanced, progesterone concentration increased again.
Discussion
Reproductive variables
Inclusion of 25% coffee pulp in the concentrate did not modify the onset of estrus, which occurred 31.61 h after removal of the CIDR in the control treatment and 34.94 h in the coffee pulp treatment. This is similar to the 36.2 h reported by Cleeff et al. (1998), but greater than the 26.5 h after CIDR removal reported by Godfrey et al. (1999).
The lower fertility in ewes fed 450 g concentrate with 25% coffee pulp may possibly be due to the 0.572% caffeine in coffee pulp used in this study. It is corresponding to 643.5 mg consumption of caffeine per day. Caffeine is a methylxanthine, which is absorbed rapidly in humans and crosses into the placenta. In women, it has been observed that when less than 100 mg caffeine is consumed in early pregnancy (6 to 12 weeks), there is a higher risk of a miscarriage (Cnattingius et al., 2000) and losses of fetuses after 20 weeks of pregnancy (Hammer et al., 2005). After the first diagnosis of pregnancy on day 30 and suspension of coffee pulp in the diet, there were no more embryo or fetus losses. The same ewes pregnant at 30 days were diagnosed pregnant at 60 days and gave birth.
Feeding ewes with coffee pulp during the first 25 days of gestation did not modify birth weight. In this respect, Hammer et al. (2007) and Clausson et al. (2002) found no differences in birth weight or gestation time between women who consumed coffee with caffeine and those that consumed decaffeinated coffee. Bracken et al. (2003) observed that consumption of moderate amounts of caffeine does not reduce birth weight, but daily consumption of more than 600 mg of caffeine was detrimental. In this experiment, no differences in birth weight were found, possibly because ewes were supplemented with coffee pulp only during the first month of gestation, with the greatest fetal growth occurring during the last third of gestation.
Inclusion of 0, 5, 10, and 15% coffee hulls in the total diet of dairy cow has no adverse effects on milk production (Cipriano et al., 2006). Moreover, coffee hulls have been administered as 25% of the concentrate fed to dairy cows in a proportion of 60:40 forage:concentrate. Also, ruminal fermentation (Soares et al., 2007a), digestibility and production and composition of milk (Soares et al., 2007b) have been assessed and it has been determined that their inclusion is possible in lactating dairy cows. This is the first study with animals in which it was determined that inclusion of coffee by-products, such as pulp, causes adverse effects in fertility. Usually, breeding occurs when dairy cows are still lactating. In other ruminants, such as sheep and goats, breeding happen immediately after weaning. The use of coffee pulp at 25 percent is not recommended, as it reduces the probability of pregnancy.
Oxidative status
Oxidative status has been assessed mainly during peri-partum, when susceptibility to lipid peroxidation increases (Bernabucci et al., 2005). However, little is known about oxidative state during estrous synchronization and early gestation.
The inclusion of coffee pulp increased antioxidant capacity before progestogen insertion. Antioxidant capacity can be modified mainly for three reasons: first, a greater antioxidant intake; second, a greater use of antioxidants; and third, greater enzymatic synthesis of antioxidants. In the present study ewes were in the same physiological state so the difference in antioxidant capacity may have been due to a greater antioxidant intake from coffee pulp. In this regard, Mumford et al. (2015) found that antioxidant concentrations are associated with steroidogenesis, while Sönmez et al. (2009) reported that intravaginal progesterone sponges increased lipidic oxidation, thus the antioxidant effect of coffee pulp observed before progestogen insertion was not observed in ewes with progestogen or in pregnant ewes because there are other factors in these stages that may have masked the antioxidant effect of coffee pulp.
Regarding the effect of sampling times on antioxidant capacity, it is important to emphasize that in both treatments antioxidant capacity was at its maximum the day after progestogen removal, and it decreased progressively as gestation advanced. In a similar study, Mohebbi-Fani et al. (2012) found that vitamins with antioxidant capacity (such as vitamin A, E, and C) decrease from the beginning of gestation; so early gestation involves a reduction in antioxidant capacity. Although there is no record of antioxidant capacity during the first few days of gestation, other researchers have found that it decreases a few days before parturition (Turk et al., 2013). Lipid oxidation generally follows the same trend as antioxidant capacity, tending to decrease as gestation advances, suggesting that decrease in antioxidant capacity is due to the fact that antioxidants were used to decrease lipid oxidation. More studies are needed to determine the importance of antioxidants at the onset of gestation and their probable benefits for reproductive traits.
Progesterone
Inclusion of coffee pulp did not modify progesterone concentration. In this respect, it was observed that ascorbic acid (an antioxidant) increases progesterone concentration in women who exhibit a defective luteum phase (Henmi et al., 2003). In ovariectomized sheep, administration of 20 mg caffeine did not affect the concentration of other reproductive hormones such as FSH (Follicle-Stimulating Hormone) and LH (Luteinizing Hormone). However, prolactin concentration increased with caffeine administration (Scaramuzzi et al., 1997). In the present experiment, neither antioxidants nor caffeine in coffee pulp modified progesterone concentration in ewes during synchronization and early gestation. For this reason, it is possible that the lower fertility observed in ewes supplemented with coffee pulp was due to other causes not related to a hormonal imbalance.
It is concluded that supplementation with 25% of coffee pulp did not affect the onset of estrous, estrous response, prolificacy, or plasma progesterone concentrations of ewes. However, it increased antioxidant capacity before insertion of progestogen. Even so, supplementation with 25% of coffee pulp during estrous synchronization or early gestation is not recommended, as it reduces fertility. Further research is required to determine whether lower levels of coffee pulp could cause an adverse effect on reproduction.















