Introduction
Freezing semen enables commercialization on a global scale of sperm from animals with high genetic value (Moreno et al., 2013) favoring the development of the livestock sector (Zhang et al., 2015). However, spermatozoa are subjected to thermal, osmotic and oxidative stress throughout freezing and thawing processes, which results in plasma membrane alterations that compromise fertilization (Beran et al., 2013; Sieme et al., 2015). Additionally, studies on bovine semen have shown that sperm cryopreservation reduces motility, damages plasma and acrosomal membranes and decreases mitochondrial function (Celeghini et al., 2008).
Since the beginning of bovine semen cryopreservation, egg yolk and milk-based extenders have been used to protect sperm from the detrimental effects of cooling and freezing (Layek et al., 2016) despite they can transmit pathogens and interfere with microscopic observations (Amirat et al., 2004). Additionally, egg yolk contains substances that hinder sperm metabolism and reduce motility, acrosomal integrity, and fertilizing ability (Moreno et al., 2013). Anzar et al. (2019) hypothesized that egg yolk proteins mask the innate proteins of sperm plasma membrane, while semen dilution with protein-free extend would provide an opportunity to study mammalian sperm proteomics following cryopreservation. For these reasons, it is necessary to find alternatives to improve the quality of frozen semen.
Purified low-density lipoproteins (LDL) from egg yolk can improve motility of post-thaw bull semen, and its optimum concentration in freezing media is 8% (Moussa et al., 2002). Some hypotheses regarding the protective mechanism of LDL include stabilization of membranes, replacement of membrane phospholipids lost during cryopreservation, or interaction with or binding to deleterious proteins present in bovine seminal plasma (Bergeron et al., 2004; Akhter et al., 2011) increasing membrane resistance against cold shock (Moussa et al., 2002). This occurs because the major proteins of bull seminal plasma (BSP proteins: BSP-A1 / A2, BSP-A3, and BSP- 30-kDa) bind to sperm surface at ejaculation stimulating cholesterol and phospholipid efflux from the sperm membrane. Addition of LDL to the extender helps to prevent binding of BSP to sperm and lipid efflux from the sperm membrane resulting in increased semen quality (Thérien et al., 1999; Bergeron et al., 2004).
Trehalose is another cryoprotectant utilized in freezing media (Hu et al., 2010). Several studies have shown that trehalose positively affects post-thawing semen motility and acrosomal and plasma membrane integrity (Hu et al., 2010). Viability and mitochondrial activity are increased, and DNA is kept intact when 100 mM trehalose is added to the extender (Öztürk et al., 2017). These effects have been explained by trehalose`s ability to promote dehydration (Aisen et al., 2005) or interact with membrane biomolecules (Bucak et al., 2007). Oh et al. (2012) reported that semen cryopreserved with a tris-egg yolk extender containing 7% glycerol, 4% LDL, and 20 mM taurine, hypotaurine and trehalose improved viability, membrane integrity and acrosome integrity of bovine sperm, compared with a control tris-egg yolk extender. However, limited information is available on the use of LDL and trehalose combination in bovine semen. Therefore, the aim of this study was to evaluate post-thaw quality of bovine cryopreserved semen added with centrifuged and non-centrifuged egg yolk, low-density lipoproteins (LDL), and trehalose.
Materials and Methods
Animals
Ten ejaculates were selected from five healthy and sexually mature Holstein bulls (Bos primigenius taurus) located on farms in the Province of Antioquia, Colombia. The bulls were kept under controlled management and feeding conditions. The collection procedure was supervised by a veterinarian to avoid stress and discomfort for the animals. No tranquilizers, analgesics or anesthetics were necessary because these animals were trained for the procedure.
Treatments
Commercial Triladyl® (Minitube, Tiefenbach, Germany) was used as the basic semen extender. It was prepared according to the manufacturer’s specifications and was stored at 4 to 5 °C. Five treatments were developed by adding to the extender: T1: pure egg yolk (PEY) at 20% (Waheed et al., 2012); T2: centrifuged egg yolk (CEY) at 20% v/v, prepared according to Nouri et al. (2013); T3: low-density lipoproteins (LDL) at 8% v/v, prepared according to Moussa et al. (2002); T4: trehalose (Merck, Darmstadt, Germany) at 100 mM (Uysal and Bucak, 2009); and T5: trehalose at 100 mM plus LDL at 8% v/v (TLDL).
Semen collection and dilutions
Semen samples were collected once per week for a period of ten weeks using an electroejaculator (Electrojac 6, Neogen Corp., Lansing, MI, USA). First, the bull was mechanically immobilized in a bucking chute. Then, the hair of the prepuce was cut and urination was induced by a stimulating massage. Subsequently, the preputial orifice was washed with saline solution and dried with a paper towel. Following this, rectal evacuation was performed before inserting the probe and switching on the electroejaculation device, whose voltage was increased automatically (Baiee et al., 2018). Subsequently, the ejaculate was collected in a sterile plastic sleeve and its color, volume, pH, sperm concentration, and mass motility were determined using a graduated glass collection tube, pH-indicator strips (Merck, Darmstadt, Germany), a specific spectrophotometer (Spermacue®, Minitube, St Louis, MO, USA) and a phase contrast microscope (100× magnification) (Domínguez et al., 2008), respectively.
Finally, semen samples were diluted to a final concentration of 30 × 106 spermatozoa mL-1, for each treatment (supplemented extender), and placed in an Equitainer® (Hamilton BioVet, Ipswich, MA, USA) at 4°C to be transported for 1 h to the Biotechnology laboratory of Politécnico Colombiano Jaime Isaza Cadavid (Municipality of Bello, Province of Antioquia, Colombia). The minimum requirements of semen quality to process the ejaculates were 70% normal morphology, 70% total motility and a sperm concentration of 500 × 106 semen mL-1 (Khumran et al., 2015).
Semen freezing and thawing
The extended semen samples were equilibrated at 4 °C for 1 hour in a refrigerator and loaded into 0.25 mL mini straws (IMV Technologies, L'Aigle, France), which were sealed with polyvinyl alcohol (Merck, Darmstadt, Germany) and placed on racks 4 cm above the surface of the liquid nitrogen vapor for 15 min. Subsequently, the straws were stored in liquid nitrogen (LN2). After a week of storage in the LN2 tank (−196 °C), cryopreserved samples were thawed by plunging two straws from each treatment into a water bath at 37 °C for 1 minute.
Sperm quality evaluation
Evaluation of semen quality for all treatments was carried out after dilution and transport of samples (before equilibration time) and immediately after the freezing-thawing process (Khumran et al., 2015). Post-thawing, a total of 100 semen straws (20 straws per treatment) were evaluated for seminal quality parameters.
Motility and kinetics. Sperm motility and kinetics were evaluated using the Sperm Class Analyzer (SCA®) system (Microptic S.L, Barcelona, Spain). A drop of thawed semen (7 μL) was placed on a prewarmed (37 °C) glass slide, and covered with a cover slip. A minimum of 500 spermatozoa were immediately analyzed (100×; Eclipse E200, Nikon Inc., Tokio, Japan) for total motility (TM), progressive motility (PM), average path velocity (VAP), straight line velocity (VSL) and curvilinear velocity (VCL).
Structural membrane integrity (SMI). SMI was evaluated using the Live/Dead Sperm Viability kit (Molecular Probes Inc., Eugene, OR, USA). A drop of 50 μL of thawed semen was mixed with 0.3 μL of SYBR14 at a final concentration of 6 μM (green fluorescence) and incubated for 8 min at 35 °C. Following this, propidium iodide (PI; 0.48 mM) was added to the mixture and incubated for 8 min at 35 °C. Samples of 7 μL of thawed semen were placed on a glass slide, and covered with a cover slip. At least 200 spermatozoa were counted using an Eclipse E200 microscope with HBO fluorescence (Nikon Inc, Tokio, Japan). Percentages of live spermatozoa were established (Gamboa et al., 2010).
Sperm vitality (SV) and abnormal morphology (AM). SV and AM were assessed using eosin- nigrosin stain. A drop of 20 μL of thawed semen was mixed with 20 μL of eosin-nigrosin (Merck, Darmstadt, Germany) on a slide for 15 seconds. The mixture was smeared using a glass slide and was dried on a thermal plate at 37 °C. At least 200 spermatozoa were evaluated for each test using a phase contrast microscope (Eclipse E200, Nikon, Tokio, Japan) at 400× and at 1000× with immersion oil. Spermatozoa with unstained heads were considered to be alive and spermatozoa with pink heads were classified as dead (Khumran et al., 2015). At the same time, spermatozoa with normal or abnormal structure were classified accordingly (Nagy et al., 2013).
Functional membrane integrity (FMI). FMI was evaluated by the hypoosmotic test (HOST) (Rekha et al., 2016). For this, 50 μL of thawed semen was mixed with 200 μL of a pre-warmed (37 °C) hypoosmotic solution of 5.4% sucrose (100 mOsmol/l) in Falcon tubes, and incubated at 37 °C for 40 minutes. After that, 7 μL of the incubated solution was placed on a prewarmed (37 °C) glass slide, and covered with a cover slip. A minimum of 200 spermatozoa were immediately analyzed in different fields (400×; Eclipse E200, Nikon Inc., Tokio, Japan). Reacting spermatozoa (rolled tails) were considered as sperm with intact membranes (Baiee et al., 2018). The percentage of reacting spermatozoa was calculated.
Statistical analysis
A completely randomized design was used. The normal distribution of the variables was validated through the Kolmogórov-Smirnov test to determine whether the analysis should be parametric or nonparametric. A generalized linear model (GLM) was used to evaluate the sources of variation. Fixed effects of treatment and the ejaculate nested within bull were included. In order to compare the adjusted means between treatments, a Tukey test was performed. All analyses were developed using SAS version 9.2 software (SAS Institute Inc., Cary, NC, USA; 2008).
Results
A total of ten ejaculates were processed under the different treatments. Within the statistical models used, the fixed effects of treatment and the ejaculate nested within bull were significant for all variables in the extended and thawed semen (p<0.05). Motility and kinetics results of extended semen are presented in Table 1.
Table 1 Motility and kinetics of extended semen.

TM: Total Motility. PM: Progressive Motility. VCL: Curvilinear Velocity. VSL: Straight-Line Velocity. VAP: Average Path Velocity. PEY: Pure Egg Yolk. CEY: Centrifuged Egg Yolk. LDL: Low-Density Lipoproteins. T: Trehalose. TLDL: Trehalose and LDL. The results are presented as mean ± standard error of mean (S.E.M). Different superscript letters (a, b) within columns indicate significant differences (p<0.05).
Figure 1 presents the results for morphology, vitality, and functional and structural integrity of the plasma membrane of the extended semen for each treatment before freezing.
Motility and kinetics results of thawed semen are presented in Table 2.
Figure 2 presents morphology, vitality, and functional and structural integrity results of plasma membrane for thawed semen.

Figure 1 Morphology, vitality and membrane integrity of extended semen. AM: Abnormal Morphology. SV: Sperm Vitality. FMI: Functional Membrane Integrity. SMI: Structural Membrane Integrity. PEY: Pure Egg Yolk. CEY: Centrifuged Egg Yolk. LDL: Low-Density Lipoproteins. T: Trehalose. TLDL: Trehalose and LDL. The results are presented as mean ± standard error of mean (S.E.M). Different letters (a, b; bars per treatment) indicate significant differences (p<0.05).
Table 2 Motility and kinetic parameters of thawed semen.
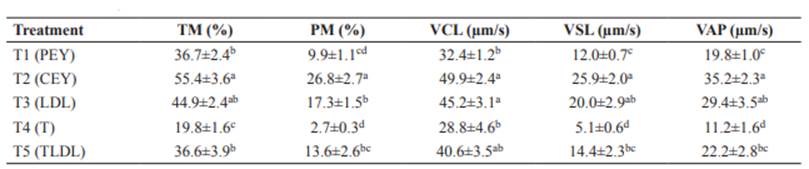
TM: Total Motility. PM: Progressive Motility. VCL: Curvilinear Velocity. VSL: Straight-Line Velocity. VAP: Average Path Velocity. PEY: Pure Egg Yolk. CEY: Centrifuged Egg Yolk. LDL: Low-Density Lipoproteins. T: Trehalose. TLDL: Trehalose and LDL. The results are presented as mean ± standard error of mean (S.E.M). Different superscript letters (a, b) within columns indicate significant differences between columns within a row (p<0.05).
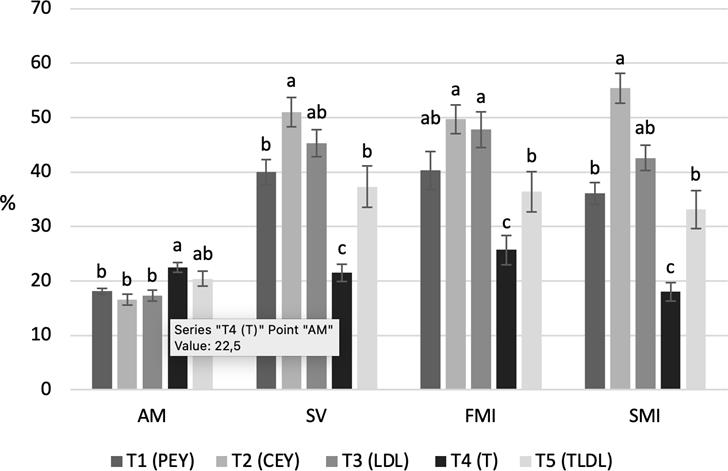
Figure 2 Morphology, vitality and membrane integrity of thawed semen. AM: Abnormal Morphology. SV: Sperm Vitality. FMI: Functional Membrane Integrity. SMI: Structural Membrane Integrity. PEY: Pure Egg Yolk. CEY: Centrifuged Egg Yolk. LDL: Low-Density Lipoproteins. T: Trehalose. TLDL: Trehalose and LDL. The results are presented as mean ± standard error of mean (S.E.M). Different letters (a, b; bars per treatment) indicate significant differences (p<0.05).
Discussion
In recent years, a demand for alternatives to conventional commercial semen extenders has arisen, since the risk of introducing exotic diseases through transport of products based on egg yolk has been recognized (Layek et al., 2016). Egg yolk can also inhibit spermatozoa metabolism and reduce their functionality (Moreno et al., 2013). Replacement of egg yolk with low-density lipoproteins (LDL) can improve post-thaw semen quality and conception rate (Anand et al., 2017). In the present study, different egg yolk separation methods, such as centrifugation and purification, were used to conserve mainly the fraction rich in LDL and eliminate those components that could generate spermatozoa alterations. Additionally, trehalose was included as an alternative to improve semen cryopreservation due to reduction of the oxidative stress induced by freeze-thawing (Hu et al., 2010).
Our results showed an effect of the additives on sperm motility and kinetics after semen dilution (Table 1). In general, CEY, LDL and TLDL favored sperm motility and speed, compared to PEY and trehalose. The addition of PEY -the most commonly used additive- reduced PM, VCL and VAP. Other studies have described that it is difficult to predict the effect of egg yolk, because there is great variability between batches due to its complex composition (Freschi et al., 2011; Pillet et al., 2011).
Integrity and functionality of the plasma membrane were negatively affected in the medium supplemented only with trehalose (Figure 1), which confirms the importance of LDL to protect the plasma membrane even in the dilution stage and during refrigerated transport prior to freezing. It is likely that, according to the action mechanisms described for LDL, stabilization of the plasma membrane is the most relevant effect in this stage (Akhter et al., 2011). No difference was observed between treatments with regard to sperm morphology in the extended semen, indicating that there were no alterations in morphology that could be mitigated differentially by any of the additives used.
For thawed semen, our results show that CEY protects motility and kinetics more efficiently than other treatments (Table 2), in addition to protecting the plasma membrane integrity (Figure 2). Although LDL had equivalent results to CEY for most parameters, this last treatment was superior with regard to progressive motility. A possible explanation is that CEY could have conserved important egg yolk components after centrifugation, which could have been eliminated during LDL purification. Egg yolk has antioxidants, such as vitamins E and B12, biotin and phosvitin (Seuss-Baum, 2007). These antioxidants have been associated with beneficial effects on cryopreserved semen of rams (Hamedani et al., 2013), buffalo (Beheshti et al., 2011), humans (Kalthur et al., 2011), and bulls (Güngör et al., 2019).
Among the CEY components, LDL lipids could prevent direct contact of sperm with ice crystals by forming a protective layer (Moussa et al., 2002), while antioxidants could reduce lipid peroxidation of the plasma membrane (Surai et al., 1998). However, according to Anzar et al. (2019), the exact sperm protection mechanism of egg yolk during the initial cooling phase is not fully understood; however, it is known that LDL sequesters the binder of sperm proteins (BSPs) in seminal plasma, which causes phospholipids and cholesterol efflux from the plasma membrane.
Current evidence suggests that LDL supplementation is one of the best options for sperm protection during cryopreservation (Peruma, 2018), and our results support this hypothesis (Table 2). It is possible that LDL was superior to PEY with regard to motility and kinetics because egg yolk contains high- density lipoproteins (HDL) that decrease sperm metabolism (Moreno et al., 2013). Additionally, it is known that egg yolk generates microbiological risk and alterations of motility, acrosome integrity and oxidative phosphorylation of sperm, and complicates the biochemical, metabolic and microscopic evaluation of semen (Wall and Foote, 1999; Moussa et al., 2002; Sariozkan et al., 2010). Amirat et al. (2010) found that LDL (8%, v/v) has simmilar or superior cryoprotective effect on bull semen in comparison to egg yolk (20%, v/v). Given that our LDL results were lower for progressive motility compared with CEY supplementation (Table 2), it may be necessary to reconsider the optimal LDL percentage. Won- Mo Cho et al. (2012) found higher sperm quality using LDL at 4% v/v.
The cryoprotective effect of LDL was also observed in combination with trehalose (TLDL), which did not differ from PEY for any parameter (Table 2 and Figure 2), and showed lower results than LDL for FMI (Figure 2). According to these, trehalose addition would not be essential to improve seminal quality when supplementing LDL. However, it has been reported that it is possible to improve post-thaw quality of ovine and bovine sperm by using LDL and trehalose during freezing (Tonieto et al., 2010; Shin-Ae et al., 2012).
Trehalose has been proven to protect proteins and membranes from dehydration (Lins et al., 2004). Although its mechanism of action has not yet been determined, it is thought that it covers layers of water; thus, preventing sperm exposure to ice crystals. This cryoprotective effect was not evident in our results; conversely, the trehalose treatment was the only one showing increased abnormal sperm (Table 2 and Figure 2) although it was used in optimal concentrations (Uysal and Bucak, 2009). The beneficial effects previously observed with the use of trehalose could be due to the simultaneous supplementation with egg yolk in the freezing medium (Hu et al., 2010; Büyükleblebici et al., 2014).
In conclusion, egg yolk could be replaced by centrifuged egg yolk or low-density lipoproteins in the freezing extender for bovine semen. However, since centrifuged egg yolk improves progressive motility of sperm and it is also easier to obtain, it could be considered as a better alternative to replace egg yolk in the freezing extender.














