Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.183 Medellín Jan./Feb. 2014
https://doi.org/10.15446/dyna.v81n183.36287
http://dx.doi.org/10.15446/dyna.v81n183.36287
EFFECTS OF FLOTATION VARIABLES ON FELDSPATHIC SAND CONCENTRATION
EFECTOS DE LAS VARIABLES DE FLOTACIÓN EN LA CONCENTRACIÓN DE ARENAS FELDESPÁTICAS
ALEJANDRO ARGÜELLES - DÍAZ
PhD. Profesor, Universidad de Vigo, España, aargu@uvigo.es
JAVIER TABOADA - CASTRO
PhD. Profesor, Universidad de Vigo, España, jtaboada@uvigo.es
FERNANDO GARCÍA - BASTANTE
PhD. Profesor, Universidad de Vigo, España, bastante@uvigo.es
MARÍA ARAÚJO - FERNÁNDEZ
PhD. Profesora, Universidad de Vigo, España, maraujo@uvigo.es
Received for review December 26th, 2012, accepted May 16th, 2013, final version June, 18th, 2013
ABSTRACT: The aim of this research was to determine the influence of certain variables (e.g., collector addition, pulp dilution, conditioning times, pH and pulp agitation speed) on the yield for feldspar sand flotation aimed at separating quartz from feldspar and to estimate the values that provide an optimum yield. The feldspathic sands were obtained from a sedimentary deposit located in the Sarreaus region (Ourense province, NW Spain).
Flotation yield was based on two parameters: grade and recovery. Each of these parameters was determined in a series of experiments in which a single parameter was varied at a time while the remainder were kept constant. The results indicate that it is possible to obtain a high flotation yield using optimum process variables. With these optimum variables, the average concentrate grade and recovery were 95.1% and 25.6%, respectively.
Key words: Mineral processing, froth flotation, flotation reagents, flotation depressants, flotation collectors.
RESUMEN: El objetivo de este trabajo es determinar la influencia que determinadas variables (como son, concentraciones del colector, dilución de la pulpa, tiempos de acondicionamiento, pH y velocidad de agitación de la pulpa) ejercen en el rendimiento del proceso de flotación de arena feldespática, utilizado para separar el cuarzo del feldespato, así como estimar los valores que proporcionan un rendimiento óptimo. Las arenas feldespáticas provinieron de un depósito sedimentario ubicado en Sarreaus, provincia de Orense al noroeste de España.
El rendimiento de flotación se basa en dos parámetros: ley y recuperación. Cada uno de estos parámetros fue determinado en una serie de experimentos en los cuales se varió cada vez una de las variables manteniéndose fijos los valores del resto. Los resultados indican que es posible obtener un rendimiento elevado en la flotación habiéndose determinado las variables óptimas de proceso. Con dichas variables óptimas la ley media del concentrado fue del 95.1% y la recuperación media del 25.6%.
Palabras clave: Procesamiento de minerales, flotación por espuma, reactivos de flotación, depresores de flotación, colectores de flotación.
1. INTRODUCTION
Feldspathic materials for the glassmaking and pottery industries are obtained from feldspathic sands. However, in order to obtain an end product of suitable quality, a series of separation tasks need to be implemented, given that these minerals are - to a greater or lesser degree - generally rendered impure by ferromagnesian minerals. The flotation process to separate feldspar from quartz was developed in the 1930s [1]. Since then, many papers have been published on the application of different collectors, reagents, depressants and other substances to the flotation of feldspars [2-14].
Feldspar in Spain is generally recovered either by means of magnetic separation or in flotation circuits.
The lack of physical, chemical and size standardization in the feldspars sold in Spain - with proportions of quartz and iron that vary from one batch to another - have forced consumers to look elsewhere for suppliers, with negative repercussions for Spanish vendors. There is also, moreover, a lack of investment in technologies and processes to improve the exploitation of mineral reserves.
Furthermore, the shortage of deposits rich in feldspathic material make it necessary to mine other types of local deposits (as described in this study), and this becomes more feasible in terms of recovery if suitable flotation techniques are used.
The main aims of this research were to determine the influence of specific parameters on the yields for feldspathic sand flotation aimed at separating feldspar from quartz (after removal of ferromagnesian minerals) and to define the values of the parameters which would optimize the flotation yield.
2. EXPERIMENTAL
2.1. Material
The mineral used for the purposes of this study was fine-grained arkosic sand with sodium-potassium feldspar, obtained from a quaternary deposit located in the Sarreaus region (Orense province, NW Spain).
Most of the feldspathic materials mined in Spain are obtained from sedimentary deposits, containing feldspathic sands composed mainly of quartz and feldspar along with other accessory minerals or impurities, such as muscovite, tourmaline, biotite, etc.
The sands used in our particular study contained biotite as an impurity, which had to be removed in a preliminary stage, prior to flotation, using high-intensity dry magnetic separators.
Fifty 5-kg samples were collected at the treatment plant after a drying process. The grain size analysis showed that 62% of the arkosic sand was less than 500 mm in diameter. The coarse sand fraction (greater than 500 mm) was 38% and the fine sand fraction (less than 63 mm) was 0.5%.
Samples were prepared and flotation tests were conducted at the mineralurgical laboratory of the University of Vigo (Spain).
The mineral was classified using an electric sieve shaker and the 63-mm to 500-mm fraction was collected. Sizes outside this range were discarded as unsuitable, since they tend to cause problems in the flotation process [15].
A high-intensity, 22000-gauss dry magnetic separator was used to separate the mica from the feldspar and quartz. This reduced the mica content of the initial sample by 95%. The mineral was then mixed and quartered.
The mineralogical composition of the 63-mm to 500-mm fraction was determined using X-ray diffraction. Chemical composition was determined using X-ray fluorescence.
Tables 1 and 2 show the mineralogical and chemical composition of the 63-mm to 500-mm fraction before and after the magnetic separation, respectively.
Analysis of the data shown in Tables 1 and 2 indicated that the micaceous minerals could be removed by high intensity magnetic separation. Magnetic separation alone decreased the magnetic mineral content of the feed to 0.1% Fe2O3 and 0.058% TiO2.
2.2. Flotation experiments
The experiments consisted of a series of froth flotation concentration tests [16], in which a single parameter was varied at a time while the remainder were kept constant, with the objective being to determine each parameter's influence on flotation yield [17].
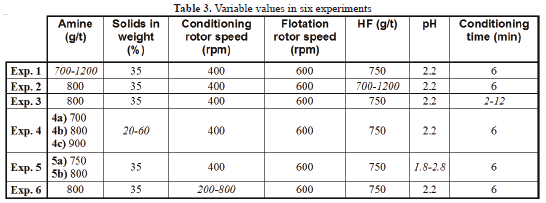
The parameters and conditions under which each experiment was conducted are summarized in Table 3. Italics indicates the range of variation for each parameter.
The flotation machine rotor, with an electronic variable speed drive, was 0.15m in diameter. The flotation cell size was 13L.
The collector used was an amine (trade name ARMAC T) - the collector typically used in feldspar flotation [18]. Hydrofluoric acid (HF) was used as a depressant regulator to prevent quartz flotation. This has the advantage of being very effective but, because this acid is corrosive, the lowest possible concentrations were used [19]. Sulphuric acid was used as the pH regulator for the pulp.
It was not necessary to use a frother, since the amine ensured a consistent foam in the tests conducted. In tests using pine oil as a frothing agent, the resulting foam was too consistent and not only impeded transport, but also prevented the eclosion necessary to free the mineral.
For each flotation test a quantity of 2 kg was used, corresponding to the non-magnetic fraction obtained in the separator. The laboratory experimental procedure is outlined as follows:
- Dilute the sample in water
- Measure pH
- Add the amine and HF
- Agitate during the conditioning time
- Measure pH and control with sulphuric acid
- Open the air inlet valve to the flotation cell
- Begin the flotation test until overflow stops
- Dry and weigh overflow and underflow.
To analyse flotation results two parameters were defined:
- Grade as a % of weight. Quotient between feldspar weight in the float and floated weight, indicating the richness of the floated feldspar and also the ideal concentration of the reagents.
- Recovery as a % of weight. Quotient between float weight and sample assayed weight, indicating the process yield.
An analysis of these two parameters defined the process yield and and indicated the ideal values of the process variables.
3. RESULTS AND DISCUSSION
Below the results obtained for each experiment are described. The effect of each variable on grade and recovery was interpreted to assess the flotation yield and to establish optimum values for the variables.
Table 3 summarizes the flotation tests, showing the values maintained when particular variables were studied.
3.1. Effects of amine addition
In this experiment (Exp. 1 in Table 3), flotation tests were implemented with varied amine additions while holding the remaining parameters constant.
Figure 1 shows variations in grade and recovery according to changes in amine addition.
Recovery rose steadily with increasing amine addition, since greater amounts of collector caused more mineral to float. Grade, however, fell after peaking at 800 g/t because increasing quantities of quartz floated as well as feldspar. In a test conducted with 2200g/t of amine, the entire sample floated, and no mineral was left in the tailings.
The optimum amine values fell within the range 750-850g/t, with mean concentrate grade above 95%. It is not clear whether a major increase in recovery would compensate for a drop in grade due to higher amine usage.
The amine adsorption mechanism is not yet fully understood. At low collector addition and low pH, surface adsorption of the collector was high for feldspar and low for silicate (quartz). This high surface affinity of the collector for feldspar can be associated with the formation of inter-molecular complexes with a high hydrophobic capacity [20].
3.2. Effects of hydrofluoric acid
In this experiment (Exp. 2 in Table 3), flotations were implemented with different concentrations of HF, with the remaining parameters held constant. Figure 1 depicts the effect of varying HF concentration.
Recovery achieved a maximum value at a HF concentration of approximately 750g/t, and then began to fall because HF has the effect of being a quartz depressant. HF also prevented the feldspar from floating, so the quantity of floating mineral fell as HF concentrations were increased.
Grade began to rise to a maximum, fell and then rose again. This happened because with low initial concentrations of HF, the quartz was depressed and did not float, with grade maintained almost constant for a HF concentration of 750-900g/t.
A slight fall occurred between 900 and 1000g/t, because the feldspar began to depress, but from 1000g/t the grade began to increase again (concentration was sufficiently high to depress feldspar and quartz). Therefore, the flotation resulted in hardly any quartz, but there was also less feldspar and recovery became increasingly lower.
A range of 750-850g/t would seem to be the optimum HF concentration, since both grade and recovery would be close to a maximum.
3.3. Effects of pre-flotation pulp conditioning time variations
In this experiment (Exp. 3 in Table 3), the influence of pulp conditioning time on flotation yield was analysed. Flotations were implemented for five different time conditions, with all the other parameters held constant.
Figure 2 shows the results obtained for the different conditioning times. For shorter times, both grade and recovery were low since there was insufficient time for adsorption of the mineral by the collector. As time increased up to six minutes grade and recovery both improved, as the collector was gradually adsorbed and implemented its hydrophobic function with the feldspar.
From six minutes, grade and recovery appeared to stabilize so the optimum time was set at six minutes.
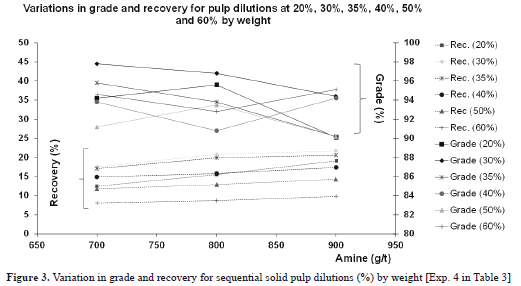
3.4. Effects of pulp dilution variations
Various dilutions were implemented in these experiments (Exps. 4a, 4b and 4c in Table 3), with the amine addition varied in each dilution. The proportions of solids by weight used were 20%, 30%, 35%, 40%, 50% and 60%. Amine concentrations were 700g/t, 800g/t and 900g/t.
Figure 3 shows the results obtained. The interpretation of behaviour at each dilution is outlined below:
- For a dilution of 20% by weight, the recovery increased with collector concentration, as the quartz also floated; grade started to fall from above 800g/t of amine, however, due to the greater presence of quartz in the float.
- For a dilution of 30% by weight, the grades and recoveries were greater than for the previous experiment, given that the proportion of solids in the sample was greater.
- For a dilution of 35% by weight, the graph obtained was similar to that of the previous case, with some small differences in recovery and grade indicating that the dilution difference was correspondingly small.
- For a dilution of 40% by weight, the graph obtained was similar to that for the 35%, with similar grades but with a gentle fall in recovery due to the fact that the solids concentration began to be significant, and the collector was therefore adsorbed with some difficulty by the mineral.
- For a proportion of 50% by weight, the grade was maintained at the preceding values; that is, it was little affected by an increase in the proportion of solids because the collector was not physically prevented from being adsorbed by the mineral. However, the collector was prevented from reaching further quantities of mineral, so recovery began to fall sharply.
- For a proportion of 60% by weight, grade was maintained at the above values for the same reasons as given above. Recovery underwent a notable fall, due to the fact that the proportion of solids was already high, so the probability of contact between the collector and mineral particles was reduced.
- A final assay conducted with 80% in solids resulted in a miniscule quantity of floating mineral. At these densities, particle clusters are very large, so the collector is adsorbed with great difficulty. Moreover, larger particles do not have the same mobility as in lower dilutions and the probability of contact with the collector is thus significantly reduced.
3.5. Effects of pulp pH variations
Two series of assays with pH variations were conducted, for two different additions of amine (750g/t and 800g/t). The results obtained are shown in Figure 4, where it can be observed that up to a value of approximately 2.2, the influence of pH on recovery was of little significance. Above this value, however, feldspar selectivity was reduced and, as a result, grade fell sharply; the recovery level also fell, but more gently.
It can be observed that from pH values above 2.2, more quartz was floated and selectivity fell in line with grade. These results would indicate that selective feldspar flotation is only possible for a narrow range of pH conditioning. The loss of selectivity at high pH values can be attributed to a reduction in the Z potentials for the minerals. For pH values higher than 3, selectivity was lost totally.
These results are similar to those for the previous series. Recovery increased slightly because collector concentration rose, whereas grade decreased because more quartz floated due to the increase in collector concentration.
3.6. Effects of pre-flotation pulp conditioning rotor speed variations
In order to determine the effects of rotor speed, six flotations at different speeds were implemented, with all the remaining parameters held constant (Exp. 6 in Table 3). Results are shown in Figure 5.
At low rotor speeds, pulp agitation was low and most mineral particles were not in suspension. Therefore, the collector was not adsorbed, and recovery and grade were consequently low.
As speed was increased, recovery and grade gradually increased, with the maximum achieved for both in the interval 400-600rpm.
At speeds over 800rpm, recovery and grade began to fall; the agitation of particles was high, causing some particles to break, resulting in a larger quantity of fine particles. These fines surrounded the other particles and prevented the collector from doing its work.
Moreover, a high level of agitation forced some of the pulp out of the cell and so a proportion of pulp was lost.
4. CONCLUSIONS
In all the assays conducted, recoveries were low, due to the fact that only a single flotation was implemented per sample. This recovery could be increased considerably by increasing amine addition, with the corresponding drawback: a large amount of quartz would be floated but the grade would be very low.
Sufficient feldspar remained for recovery in the tailings for each flotation. This could be easily recovered with successive flotations of the tailings, resulting in higher recovery rates, thereby justifying implementation in industry.
The most important factor is to ensure that variables are adjusted to obtain a high grade in a single flotation - as has been done in our research - since achieving high recovery rates over several successive flotations of the tailings is a less complex process.
An analysis of the results indicated that the optimum variables for the process were those summarized in Table 4.
With these optimum values the mean concentrate grade and mean recovery rates obtained for our flotation tests were 95.1% and 25.6%, respectively.
ACKNOWLEDGEMENT
The authors acknowledge the reviewer's comments and suggestions, which contributed greatly to improving the quality of the final version of this paper. Ailish M.J.Maher provided assistance with English usage in a version of the manuscript.
REFERENCES
[1] O'meara, R. G., Norman, J. E. and Hammond, W. E., Froth flotation and agglomerate tabling of feldspar, Bull. Am. Ceram. Soc., 18 (8), pp. 286-292, 1939. [ Links ] [2] Manser, R. M., Handbook of Silicate Flotation, Warren Spring Lab. Stevenage, England, 1975. [ Links ] [3] Rao, H. K. and Forssberg, K. S. E., Feldspar flotation: theory and practice, In: Selected Topics in Mineral Processing (Eds. A. Gash, R. Pradip Kumar and S. Venkatachalam), Port City Press, Baltimore, pp. 86-117, 1995. [ Links ] [4] Shehu, N. and Spaziani, E., Separation of feldspar from quartz using EDTA as modifier, Minerals Engineering, 12 (11), pp. 1393-1397, 1999. [ Links ] [5] Demira, C., Bentlib, I., Gülgönülc, I. and Çelikd, M. S., Effects of bivalent salts on the flotation separation of Na-feldspar from K-feldspar, Minerals Engineering, 16 (6), pp. 551-554, 2003. [ Links ] [6] Bayat, O., Arslan, V. and Cebeci, Y., Combined application of different collectors in the flotation concentration of Turkish feldspars, Minerals Engineering, 19 (1), pp. 98-101, 2006. [ Links ] [7] Gougazeh, M., Evaluation and beneficiation of feldspar from arkosic sandstone in South Jordan for application in the ceramic industry, American Journal of Applied Sciences 3 (1), pp. 1655-1661, 2006. [ Links ] [8] Vidyadhar, A. and Rao, K. H., Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system, Journal of Colloid and Interface Science, 306 (2), pp. 195-204, 2007. [ Links ] [9] Mantilla, C., Pedraza, J. and La Verde, D., Application of zeta potential studies in the development of an alternative process for the flotation of feldspar minerals, DYNA, 75 (154), pp. 65-71, 2008. [ Links ] [10] Aksay, E. K., Akarj, A., Kaya, E. and Cocen, I., Influence of fatty acid based collector on the flotation of heavy minerals from alkali feldspar ores, Asian Journal of Chemistry, 21 (3), pp. 2263-2269, 2009. [ Links ] [11] Repková, M., Botula, J. and Repka, V., Application of the froth flotation for feldspar ores treatment, In proceeding of 11th International Multidisciplinary Scientific Conference, At Albena, Bulgaria, Vol. 1, pp. 1077-1082, 2011. [ Links ] [12] Hacifazlioglu, H., Kursun, I. and Terzi, M., Beneficiation of low-grade feldspar ore using cyclojet flotation cell, conventional cell and magnetic separator. Physicochemical Problems of Mineral Processing, 48 (2), pp. 381-392, 2012. [ Links ] [13] Heyes, G. W., Allan, G. C., Bruckard, W. J. and Sparrow, G. J., Review of flotation of feldspar. Transactions of the Institutions of Mining and Metallurgy, Section C: Mineral Processing and Extractive Metallurgy, 121 (2), pp. 72-78, 2012. [ Links ] [14] Zhang, Z., Feng, Q., Wang, W., Xu, L. and Hunag, Y., Adsorption characteristics of feldspar and quartz in anion-cation collector. Zhongnan Daxue Xuebao (Ziran Kexue Ban)/Journal of Central South University (Science and Technology), 44 (4), pp. 1312-1318, 2013. [ Links ] [15] trahar, w. j., The selective flotation of galena from sphalerite with special reference to the effects of particle size, International Journal of Mineral Processing, 3 (2), pp. 151-156, 1976. [ Links ] [16] Hadler, K., Aktas, Z. and Cilliers, J. J., The effects of frother and collector distribution on flotation performance, Minerals Engineering, 18 (2 SPEC. ISS.), pp. 171-177, 2005. [ Links ] [17] Shimoiiza, J. and Nakatsuka, K., Study on the separation of feldspar from quartz by flotation on activation with FH, Journal of Mining and Metallurgical Inst. of Japan, 80 (918), pp. 1054-58, 1964. [ Links ] [18] Sekulic, ., Canic, N., Bartulovic, Z. and Dakovic, A., Application of different collectors in the flotation concentration of feldspar, mica and quartz sand, Minerals Engineering, 17 (1), pp. 77-80, 2004. [ Links ] [19] Paulson, E. G., Reducing fluoride in industrial wastewater, Chemical Engineering (New York), 84 (22), pp. 89-94, 1977. [ Links ] [20] Vidyadhar, A., Rao, K. H. and Chernyshova, I. V., Mechanisms of amine-feldspar interaction in the absence and presence of alcohols studied by spectroscopic methods, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 214 (1-3), pp. 127-142, 2003. [ Links ]