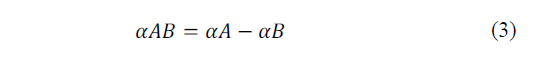1. Introduction
Parallel to the rapid global population growth has increased the need to develop new energy sources whose characteristics allow them to be environmentally friendly without losing their practical qualities and technical efficiency. According to Ožbolt, Kitanovski, Tušek and Poredoš [1], Hermes and Barbosa [2] and Uchida et al. [3], most refrigeration systems are currently based on gas vapor compression refrigerant. One of the biggest concerns regarding this type of refrigeration is its direct contribution to the greenhouse gas effect, however, despite its low cost and high reliability, steam compression cooling does not significantly affect global warming compared to other cooling technologies that work with electricity. On the other hand, the considerable noise generation of these compressors can make them unsuitable for certain tasks sensitive to auditory disturbances.
In the study by Hermes and Barbosa [2] and Boukai et al. [4] there is a tendency in current research to implement technology forms with minimal negative impact on the environment; in our case, the development of alternative cooling systems, where significant advances in the field of thermoelectric cooling stand out, and whose approach is to reduce the thermal resistance between the heat transfer medium, generally air, and the thermoelectric material.
In the study by Jakhar, Baheti, Gurjar and Sharma [5], they define "thermoelectricity" as a derivation of thermodynamics incorporated into electricity, the best known phenomenon is the flow of electrons generated by applying heat at the junction of two different materials, if two wires of different material (thermocouple circuit) are joined at both ends, and one of the joints is maintained at a higher temperature than the other, a voltage difference arises that causes an electric current to flow between the hot and cold junctions. The generated current can be increased by using semiconductors instead of metals, and a low power in watts can be achieved with efficiency of up to 6%. When a current is passed through a circuit composed of different materials whose joints are at the same temperature, the reverse effect occurs. In this case heat is absorbed from the union, emitting heat at one end, and cold at the other. The latter is called the Peltier effect [6].
With the evolution of semiconductors and the incorporation of thermoelectric devices in the market, Peltier technology has experienced important advances in recent years. In the study by Dell, Capozzi, Xia, Venkataraman, & Campos [7] and Nonoguchi et al. [8], thermoelectric refrigeration is applied in different areas such as medicine and in scientific equipment where a high precision temperature control is necessary; however, there is a predominance of traditional vapor compression systems in everyday devices and instruments such as portable refrigerators and air conditioners for domestic use [9].
Currently, thermoelectric refrigeration is used in medicine, scientific equipment and other devices where a high precision temperature control is necessary as denoted in the study by Dell et al. [7], Nonoguchi et al. [8] and Lan and Ren [10]; however, its incorporation into more common elements, such as air conditioners for domestic use, portable refrigerators, among others, is still strongly hindered by the predominance of traditional vapor compression systems [9].
It highlights certain characteristics of this type of refrigeration that place it on conventional refrigeration, such as its compact size, low weight, absence of removable mechanical parts, and ease of exchange between hot mode and cold mode among the most outstanding ones [11]. The main objective of the present investigation is to explain the operation of a thermoelectric system and evaluate its applicability from a general determination and comparison with respect to other cooling methods.
The object of this study was a scientometric review of the Peltier effect in literature by projecting it into an application in residential refrigeration systems. Motivated by the practical interest in the generation of alternative energy, this study addresses the characterization of a Peltier cell theme. This study is organized in 5 chapters as follows: the scientometric analysis, the thermoelectric fundamentals, the thermal driving, the methodology and the conclusions.
2. Scientometric analysis
Albeit the systematic revision techniques of literature- RSl come from the health performed researches [12-14]. There is a growing trend in the implementation of these techniques in the different ambits of knowledge, like engineering [15]. This study has taken [16] as a reference, where there is a RSL methodology, implemented from the correlation of two ambits (health and engineering). Three phases were proposed in this study: 1) definition of search parameters, 2) Information identification and filtering starting from the specialized data sources, and 3) results submission and analysis. In [16] the data submission was set in two categories (scientometric and technical). However, this proposal focused solely in the analysis of variables from the scientometric approach.
For the phase of search parameters definition, the following hypothesis was proposed: “Peltier effect is a research field that is moving forward more and more, because its implementation in refrigeration systems with thermoelectric energy generates low CO2 emissions, producing a positive environmental incidence.” Key words were identified from this hypothesis. For the identification and filtering phase, a search string was set starting from the key words (“thermoelectric energy” and “Peltier effect” and not “Seebeck effect” and not “Joule effect”), said string was used in the specialized database Science Direct, and articles, proceedings, and books were downloaded that fit the search criteria. With the collected information from the different sources, a data acquisition matrix was made, that documented different scientometric variables (year of publication, journal, document typology, quartile of the journal, country of publication of the journal, among others). Finally, the results were submitted in a consolidated manner, with graphics, and its analysis contributed to the proposal of results.
The measurement of the degree of scientific development lies in the quality and quantity of publications made on the subject in question, by making a scientific analysis in one of the most recognized databases worldwide as it is Science Direct [17], we can find the results shown in Fig. 1.
The year 2017 has a high publication trend, it is the year with the highest number of publications with 247 of these, followed by 2015 with 200 and 2016 with 194.
3. Thermoelectric fundamentals
According to Yilbas and Sahin [18], thermoelectric energy generation is operated by a combination of other physical phenomena found in the effective operation of a Peltier cell thermoelectric system, as mentioned by Lineykin and Ben-Yaakov [19], however this research is limited to the mention of three of them as presented below:
3.1. Joule effect
In the study by Julio-Betancourt and Hooton [20], it is defined as the interaction between an electrical phenomenon and the conduction of electric current where a term phenomenon associated with the heating of a conductor through which current flows is associated. Matter offers some resistance to the movement of electrons, which give kinetic energy to the environment in successive collisions. This energy provided by the electrons dissipates in the form of heat (eq. 1).
Where: Q is the heat energy produced by the current, I is the current intensity that circulates, R is the electrical resistance of the conductor and t is the time.
3.2. Seebeck effect
Thomas J. Seebeck discovered that in a circuit formed by two homogeneous different metals, A and B, with two unions at different temperatures, T and T + ΔT, an electric current flow J is established, or, if the circuit is a thermal electromotive force (ftem) EAB that depends on the metals used in the joint and the temperature difference between the two joints. The diagram of the mentioned configurations is shown in the Fig. 3.
The relation between the item, EAB, and the temperature difference between the joints ΔT, defines the Seebeck coefficient in eq. (2)-(3).
Where: αA and αB are respectively the absolute thermoelectric powers of A and B and are characteristics of each metal.
In general, αAB is not constant, but depends on the temperature T [21].
3.3. Peltier effect
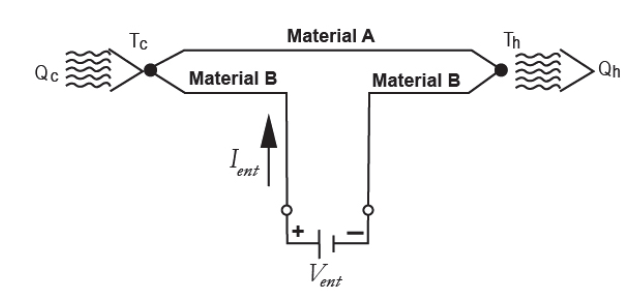
The Peltier effect consists of the cooling or heating of a connection between two different conductors when an electric current passes through them and which depends exclusively on the composition and temperature of the joint. Fig. 4 shows the scheme of the circuit [6].
The heat power exchanged at the junction between A and B is given by eq. (4)-(5).
Where: πAB is the Peltier coefficient, which is defined as the heat exchanged in the junction per unit of time and current flowing through it, J is the electric current flow, ΔT the absolute temperature difference between AB and αAB the Seebeck coefficient.
Therefore, when connecting the manufactured cell to a DC power supply, the absorbed power corresponds to one term by the Joule effect and another due by the Peltier effect (eq. 5). From eq. (6)-(7) the absorbed power is determined and presented in eq. (8).
Where: P ent is the power supplied by the source.
Internally, the Peltier cell has highly contaminated semiconductor elements and is electrically arranged in series using copper conductors. To insulate the copper conductors from the dissipater, a ceramic plate that functions as an insulator is added between them (Fig. 5).
A polarization as shown in Fig. 6 is distributed along each semiconductor element of the cell, that is, each semiconductor element has a potential difference proportional to the input polarization. For this reason, the majority carriers, weakly bound electrons, migrate towards the positive side of each of their ends in the N-type

Source: Sandoval et al. [23]
Figure 5 Peltier cell cross section showing semiconductor and temperature dissipating elements.

Source: Sandoval et al. [23].
Figure 6 Compression and rarefaction of charge carriers near the semiconductor metal junction in a Peltier cell.
semiconductor elements, due to the attraction of charges of different sign. While the majority carriers, holes of the semiconductor elements P, migrate toward the negative terminal that is at each of its ends. This absence of charges in each semiconductor element near the metal-semiconductor junction causes a rarefying of charges and the consequent temperature drop in the surrounding area. On the other hand, the compression or accumulation of carriers near the semiconductor metal junction in the lower part of the semiconductor elements in Fig. 3 causes a rise in temperature. This behavior allows us to affirm that, if we invert the polarity of the power supply, the cold face will now warm up and the hot face will suffer a drop in temperature.
4. Thermal driving
Finally, there is an internal work that is due to the thermal conduction determined by the Fourier law. It establishes that the heat transfer rate per conduction in a given direction is proportional to the normal area of the heat flow direction and to the temperature gradient in that direction. As it is an internal work this term does not have to be taken into account in eq. (9). Therefore, the heat that flows from the hottest focus to the cold per unit time for each element is:
Where: k i is the coefficient of thermal conductivity of each element per unit length through section unit, A is the section normal to the direction of flow, l the length of each element and ΔT the temperature difference in the ends of the element, n is the number of elements that make up the cell [24].
Finally, the thermal conductivity of the cell is defined as the sum of the contributions of each element (eq. 10).
5. Methodology
The Seebeck and Peltier effects are qualitatively different and were discovered separately. However, today they are understood as two aspects of the same phenomenon and receive a unified theoretical treatment. As is well known, when in a thermodynamic system there is simultaneously a thermal flux (associated with a temperature difference ΔT) and an electric charge flux (associated with an electromotive force Δε, there will be an entropy production, which in the linear approximation will be written as eq. (11):
Where: J Q is the heat flow, I is the electrical current (load flow) and T is the average temperature of the system.
When these two independent contributions to entropy are given, there must be a linear relationship between the flows and the different forces. Moreover, the heat flow will be coupled with the charge flow in such a way that:
Solving the coefficient matrix we have left (eq. 12-13):
Consider a system that obeys the previous coupled equations. When the electrical intensity tends to zero, but there is a temperature difference, from the second of the equations it is concluded that an electromotive force will appear, given by eq. (14)-(15):
According to Xu, Gan and Zhang [25], this phenomenon is called the Seebeck effect, where the coefficient α measures the intensity of the effect and is called the thermoelectric power of the material (Seebeck coefficient). Now, if a current circulates through a system with thermoelectric capacity, due to the coupling of flows, a temperature difference will appear, it is the opposite effect to the previous one, the Peltier effect.
When the current appears, the temperature difference increases, until a steady state is reached, where the total heat flow in the system becomes zero. Substituting that condition in the previous equations is a linear relationship between the temperature difference applied, this makes the intensity that runs through the system as given by eq. (16)-(17):
Where: β * I is the Peltier coefficient associated with the system [26], L11, L12 and L22 are called kinetic coefficients and are properties of the medium such as electrical conductivity, thermal conductivity, etc.
Applying the above according to Block and Walker [27] and in the absence of a magnetic field, the Onsager theory indicates that L12 = L21.
5.1. Peltier's cell and its general equations
A typical thermoelectric module consists of two thin coats of ceramic material containing an array of N and P type blocks of doped semiconductor material (e.g., bismuth-telluride), as shown in Fig. 7. Excess of electrons whereas type P blocks have shortage of electrons. A pair of blocks N and P form a thermoelectric pair, and in turn, thousands of these pairs are contained by a thermoelectric module.
When the electrons move from P to N through an electrical conductor, they jump to a state of higher energy and absorb the energy from their surroundings (cold side). The opposite happens when they move from N to P, where the fall to a lower energy state causes a release of energy to the surroundings (hot side). By reversing the polarity of the current, the direction of heat transfer changes [28].
There are several effects that occur within a Peltier cell, being able to enunciate the Peltier, Thomson and Joule effects, as denoted in the study by Patterson [30] and Chen, Yan and Wu [31]. In a given temperature range, the heat flow produced by the circulation of the electric current with variation of temperature, that is, the Thomson effect, can be neglected. Taking into account the above, when applying a potential difference on the cell, a heat transfer per unit time on the hot face will be produced equal to eq. (18):
Where: TC is the temperature of the hot side.
For the same purpose, the heat absorption per unit time on the cold side will be given by eq. (19):
Where: TF the temperature of the cold side.
On the other hand, if the losses per unit of time due to Joule effect are considered, which are distributed half for each side, these will be expressed by eq. (20):
Where: R is the electrical resistance of the Peltier cell.
The temperature difference between both sides will produce a thermal conduction effect between the hot side and the cold side, quantifiable as eq. (21):
Where: RTH represents the thermal resistance between the hot side and the cold side.
The net calorific flow absorbed by the cold side will be given by eq. (22)-(23):
While the heat given and that must be dissipated through the hot side will be equal to eq. (24)-(25):
Applying the first principle of thermodynamics, it will be found that the power supplied will be the difference between the heat fluxes of dissipation and absorption, as given by eq. (26)-(27):
What is equivalent to the electrical power needed to drive the device (Power = Intensity x Voltage). The supplied power is then converted into two heat sources; the thermoelectric heat flow, proportional to the electrical intensity, and the heat generation by Joule effect, proportional to the square of the intensity. According to Mannella, La Carrubba and Brucato [32] for refrigeration operations, the heat injected to the hot side must be dissipated by a sump. Not being so, the temperature of the hot side will increase and, consequently, the cooling performance will be reduced. This fact is illustrated in eq. (27), at a fixed electrical power, the temperature difference is constant; By increasing the temperature of the hot side, the temperature of the cold side will become higher.
5.2. The efficiency of thermal energy
The thermal energy efficiency of a thermoelectric device can be related to the so-called dimensional figure of merit ZT, which is directly proportional to the electrical conductivity and the Seebeck coefficient of the materials in the thermoelectric pair and inversely proportional to the thermal conductivity [2]. It is given by the eq. (28):
Where: σ is the electrical conductivity and K is the thermal conductivity.
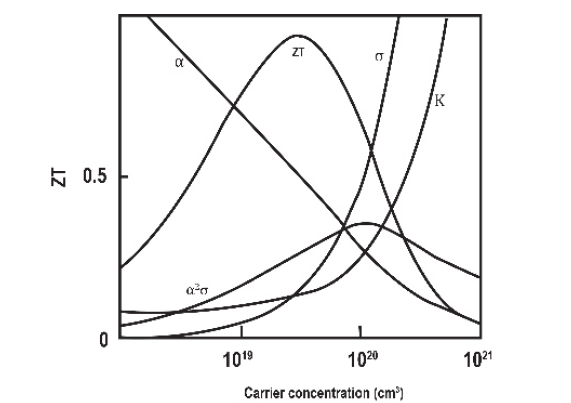
These three transport parameters depend on each other as a function of carrier concentration, and band structure, among others; as illustrated in Fig. 8.
According to Fig. 8, α and σ generally vary in a reciprocal way making any improvement in the merit figure ZT difficult. In addition, the electrical conductivity and the Seebeck coefficient are inversely related, so, generally, it is not possible to increase the thermoelectric power above a particular optimal value for bulk material. However, ideal thermoelectric materials would have a high electrical conductivity to allow the conductivity of electricity, which would create a potential difference across the sample, and a low thermal conductivity to maintain the temperature gradient between the hot and cold sides.
Most materials exhibit a correlation between electrical and thermal conductivity. A material that conducts electricity well, such as metal, also conducts heat well, and a material that functions as a thermal insulator, such as glass or ceramic, also functions as an electrical insulator [8]. The history of thermoelectric plants can be characterized by the progress in increasing the ZT, as shown in Fig. 9.
Source: Elsheikh et al. .
Figure 8 Maximize ZT involves thermal conductivity and the Seebeck effect with electrical conductivity.
In Table 1 different materials with high ZT values can be observed. Currently, the best thermoelectric materials have ZT values around 1.0 [11].
5.3. Comparative thermodynamic study of the Peltier effect with other cooling technologies
In the study by Ožbolt et al. [1] and Snyder and Ursell [34] it is defined the CR (performance coefficient) of a real cooling system εs, as the radius between cooling capacity and energy consumption (eq. 29):
Where: Q F = K(T e -T i ) in steady state.
For an ideal refrigerator, CR depends solely on the temperatures of the external environment, T e and internal, T i as indicated in Fig. 4, being calculated as indicated by eq. (30):
If we assume that the cooling device operates ideally between the hot end and the cold, T C and T F , respectively, as shown in Fig. 10, the CR, considering the thermal losses due to external irreversibilities (e.g. heat transfer with finite temperature difference in the heat exchanger), is calculated as follows (eq. 31):
where ε ii is the CR of an ideal cooling device operating with real heat exchangers, where "ii" means "internally ideal".
The second law of efficiency associated with internal irreversibilities is calculated from eq. (32):
Analogously for external irreversibilities (eq. 33):
Therefore, the second law of total efficiency for the entire refrigeration system is given by eq. (34):
Additionally, the entropy generation rate due to thermodynamic losses in the refrigerating device can be calculated by means of the following entropy balance considering the temperatures of the extremes (eq. 35):
Where: Sg is the entropy generation rate (W/K).
The entropy generation number, N S is calculated with the following expression (eq. 36):
Based on eq. (29), (35)-(36) it can be shown that the CR of a refrigerator system is as follows (eq. 37):
In the above equation, the term T C N S is an equivalent temperature difference that takes into account the irreversibilities that take place in the refrigerator.
We should note that ε s →ε ii , N S →0 and ε s →ε c and both, ∆T F and ∆T C tend to zero. According to the above it can be inferred that the temperature differences in the hot and cold ends of a refrigerator and the internal limit of the entropy generation rate, cause a deviation between εs and its ideal counterpart, ε c thus reducing the efficiency of the system.
Based on the previous comparison methodology, the thermodynamics of three types of portable refrigerators were compared: Peltier, Stirling and Steam Compression. Fig. 11 compares three CR: the real, the ideal internal and the ideal for the four cooling systems under analysis, for temperatures of 21 and 32 ºC. It is observed that, for an ambient temperature of 21 °C, the thermoelectric cooler showed the lowest CR. At 32 °C, the bars shrink slightly for all systems except for the thermoelectric system, that is, the influence of the ambient temperature on the CR was not significant, since the temperatures of both extremes, cold and hot, varied dramatically.
In addition, we should note that the ideal CR of the thermoelectric cooler was approximately double than that of the other systems because it generates the smallest temperature difference between the hot and cold surroundings.
However, since the cooling capacity of the thermoelectric system was the lowest per input power unit, its total thermodynamic efficiency was the lowest, which can be seen in Fig. 12. This value means that the internal irreversibilities in the thermoelectric module can be very high. In fact, this, combined with the comparatively high value of its internally ideal CR, confirms the need to improve the thermoelectric properties of the thermoelectric cooler.
It can also be inferred according to Hermes and Barbosa [2] and analyzing the behaviors shown in Fig. 12, that the external efficiencies of direct expansion refrigeration systems (vapor compression) are higher than those calculated for cooling systems by Indirect expansion (thermoelectric and Stirling), suggesting that the increase in the performance of emerging refrigeration technologies is based, among other things, on the design of more efficient heat exchangers.
It can also be inferred according to Hermes and Barbosa [2] and analyzing the behaviors shown in Fig. 12, that the external efficiencies of direct expansion refrigeration systems (vapor compression) are higher than those calculated for cooling systems by Indirect expansion (thermoelectric and Stirling), suggesting that the increase in the performance of emerging refrigeration technologies is based, among other things, on the design of more efficient heat exchangers.

Source: Hermes and Barbosa [2]
Figure 12 Thermodynamic efficiency (second law): (a) 21 ºC, (b) 32 ºC
6. Conclusions
The use of the Peltier effect as an alternative method of refrigeration does not seem to be able to displace, in the near future, the dominance of other traditional refrigeration media such as vapor compression. Taking into account the thermodynamic performance, thermoelectric devices are not at the same level as other refrigerators, so significant improvements are required that attack or reduce external and internal irreversibilities. However, its applications in everyday life are increasing as interest grows in the adoption of cleaner and quieter technologies, which is why we expect new research bursts around the theme, that seek to optimize the qualities of this technology, which, although it is old, it provides a series of characteristics that are usable and highlight it on the most known methods, which stand out, its ecological character, silent and more precise in the control of the temperature. The inclusion of thermoelectric technology is favored by the growing importance of computers in modern society, in which the use of thermoelectric plants has been implemented to prevent overheating.
It is necessary to increase the figure of merit ZT of the thermoelectrics and the ratio of the heat exchangers with the Peltier effect. It should be noted that an outdated technology with such low efficiency and performance, becomes important in an era full of challenges and difficulties, however, the environmental problems and technological boom are directing us towards the preference of technology as provided by the devices that work by Peltier effect, solving new needs that perhaps the most traditional cooling methods cannot respond to.