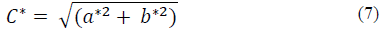1 Introduction
The compound astaxanthin is known to be a carotene belonging to the group of xanthophyll [1], because at its ends it has a hydroxyl group (OH) and keto group (CO) [2], which together with its 13 double bounds give it its great antioxidant property, its consumption is beneficial for the prevention of degenerative macular diseases, cancer, cardiovascular diseases, among others [3]. However, thanks to its chemical structure, this molecule is highly sensible to high temperatures, oxidation and light stress degradation. Additionally, it is a water insoluble molecule and has low solubility at room temperature, causing the bioactive compound to not present a good bioavailability [4].
Due to the astaxanthin’s liposoluble characteristic, it has been difficult to use in the food industry when wishing to incorporate it into hydrophilic matrices; therefore, the industry has been interested in the production of emulsions of the oil-in-water (O/W) type to be able to take advantages of these types of compounds [5]. To formulate this type of emulsions, a surfactant oil and water are mainly required [6,7]. The particles of bioactive compounds in dispersions are stabilized by an emulsifier or a mixture of emulsifiers; the latest are active surface molecules that must be absorbed into the two-phase interface, decreasing the interfacial tension and avoiding the aggregation and re-coagulation of particles [8-10].
It has been established that producing O/W emulsions supersaturated with astaxanthin improves the absorption, being considered one of the best formulations to increase the bioavailability of the astaxanthin molecule [11].
The macromolecules have a micelle size in a range of 100 nm - 100 µm [11] and can be formulated with food-grade ingredients using simple processes such as mixing [12-14]. They present a physical stability, but are thermodynamically unstable, especially when they are under stress conditions, such as gravitational separation, aggregation, and Oswald ripening coagulation [10,11].
The known mechanisms of emulsion instability are: sedimentation, creaming, flocculation, phase inversion, coalescence, and Ostwald ripening, Fig. 1[16].
The objective of the study was to determine the physical behavior of an oleoresin macroemulsion of astaxanthin, by determining viscosity at different temperatures, size of micelles and the variation of color over the time.
1. Methodology
2.1. 2.1. Materials
The astaxanthin oleoresin (10%) extracted by supercritical CO2 technique was donated by the Company Atacama BioNatural Products S.A. (Iquique, Chile). The propylene-glycol was purchased from Winkler Ltda. (Santiago, Chile). The sucrose (Brand IANSA) was obtained from a supermarket in the city of Antofagasta; and the habo-monoester P-90 surfactant was purchased from Compass Foods (Singapore, Republic of Singapore).
2.2. Preparation of macroemulsion
The macroemulsion was made from two phases, one liquid (constituted by propylene-glycol and water in a ratio of 2.50 to 2.75:1) and one solid containing sucrose and habo-monoester P90 in a ratio of 18.50 to 20.00:1. The phases were mixed and heated to T = 40 ± 5°C until the complete dissolution of sucrose using a magnetic stirring with temperature sensor model MS400 (Hilab Innovation System, China). The solution was then stirred by 5 minutes at 35000 rpm in a ultraturrax model Pro-200 (Proscientific, USA); subsequently 0.8 g of oleoresin astaxanthin (10%) was added and it was homogenized again in the ultraturrax with previously mentioned parameters, keeping the sample always in a container cover with ice in its external part. Finally, ultrasound model CP130 (Cole Parmer, USA) was applied for 20 total minutes, applying 10 minutes, followed by a pause of 5 minutes and applying 10 more minutes, with an amplitude of 60 and oscillations every 6 seconds keeping the emulsion cold, using a recipe with ice.
2.3. Microscopy and micelles measurement
The macroemulsion was observed for 8 days as the peak time of stability with a prism of 100x magnification through the optical microscope model CX31 (Olympus, Japan); for this, a sample without dilution and a diluted sample were prepared, for which 20 μL of the macroemulsion was taken, diluted in 500 μL of distilled water, mixing the solution three times with the micropipette at room temperature (T = 20 ± 4.0° C). The size of the largest micelles present in the emulsion was determined, selecting the 10 with the largest field size observed by the microscope eyepiece, which were measured with the Software Micrometrics SE-Premium coupled to the camera model U-TV0.5XC-3 (Olympus, Japan). Each sample was determined with 3 fields taken at random from the emulsion.
2.4. Viscosity measurements
The viscosity of the macroemulsion was determined in a model viscometer. RVDV-II+ (Brookfield Engineering Lab., USA), using its adapter accessory for small sample and the spindle S-21. A speed range was used that varied from 6 to 100 rpm and different temperatures (5, 10, 20, 30 ° C), which were obtained through a thermoregulated ultrasound bath model UC-30A (Biobase, Germany), without the application of ultrasound. For each combination of rotation-temperature, 10 measurements were made every 30 s.
To describe the type of fluid to which the macroemulsion belongs, the equation of the power law (eq. (1)) was used; with support from the Statgraphic® Centurion XVI program, using linear regression between shear rate and shear stress. In the same way, the coefficient of determination R2 (eq. (2)) and the errors were determined: chi-square reduced χ2 (eq. (3)), sum of square of errors, SSE (eq. (4)) and the root of the square mean error, RSME (eq. (5)). These parameters can be described in equations from as (2) and (5) [16].
Where τ (Pa s) is shear stress, K (Pa sn) is the consistency index, γ ̇ (s-1) is shear rate and n (dimensionless) is the flow behavior index.
Where SS exp,i is the experimental shear stress found in any measurement and SS pre,i is the shear stress predicted for the measurement under consideration. N and n are the number of observations and the number of constants respectively.
2.5. Chromatic measurements
The chromatic coordinates of the macroemulsion were determined through the CIEL*a*b* system with the Colorflex color model 45°/0° (Hunterlab, USA) with daylight (10°/D65) and a port size of 0.75 inches, calibrated with the black and white tile provided by the equipment. The parameters L*, a* and b* indicate the trend towards white/black, red/green and blue/yellow, respectively. For each sample, 10 measurements were made and each of the samples was made in triplicate, for a period of 32 days, taking day 0 on day 2 after the emulsion was elaborated, waiting for it to stabilize.
Where
ΔL=L* 2 −L* 1 Luminosity difference
Δα=α* 2 −α* 1 Red - green difference
Δb=b* 2 −b* 1 Blue - yellow difference
The subscripts 1 and 2 serve to designate the two moments of the determination of the colors (initial and final) between which the color difference ΔE is calculated. In addition, the intensity of the color chroma (C*) was calculated with the eq. (7) and hue tone (h°) with eq. (8).
Where α* represents chromatic coordinate red/green and b* is the chromatic coordinate blue/yellow.
3. Results and discussion
3.1. Micelle stability
The values corresponding to size of the micelles of the emulsion were 1.46 ± 0.73, 1.83 ± 1.00, 2.00 ± 0.63 and 2.31 ± 1.38 μm for days 0, 2, 6 and 8 respectively, indicating an arithmetic average increase in size through time; Nevertheless, there were no significant differences for (p <0.05), since, although some micelles showed a slight increase in size, others retained it, which is observed from the significant differences in each population, which reached values of coefficients of variation of: CV = 50.00; 54.64, 31.5 and 59.74, respectively.
Fig. 1.A shows the largest micelle size obtained for day 0 (3.10 μm), while Fig. 1.B shows the largest micelle size obtained for day 8 (8.27 μm), demonstrating that there was an increase in size in some micelles. This can be due to the phenomenon of flocculation and coalescence that can be observed in Fig. 1.C. The latter would explain the variation in micellar diameter of the macroemulsion over time, probably caused by the continuous movement to which micelles are subjected and the concentration of non-absorbed polysaccharides that contributes to flocculation [17]. The presence of these physical phenomena leads to a progressive instability in the emulsion in a slow manner, causing a decrease in the bioavailability and dissolving capacity of the astaxanthin molecule in aqueous matrices.
The micelles that were obtained had sizes greater than 200 nm in diameter; therefore, according to the micelle size, the elaborated emulsion would be categorized as a macroemulsion, which present micellar values between 100 nm and 100 μm [18].
3.2. Viscosity stability
In Fig. 3, the mean values of the shear stress (SS) versus the shear rate (SR) for the 10% astaxanthin oleoresin emulsion at different temperatures are observed. As shown in Fig. 3, the temperature has a great influence on the oleoresin macroemulsion of astaxanthin at 10%, its highest variation being between 10 and 20°C, with values for its consistency index of (K) 40.11 and 3.66. Pa sn respectively; it can also be established that the oleoresin emulsion of astaxanthin had a similar variation of the shear stress versus the shear rate for the temperatures of 5 and 10°C, denoting its shear thinning property.

Source: The authors
Figure 2 Microscopy for micelle observation during the stability analysis of astaxanthin oleoresin emulsion, with 100x magnification. A) day 0, sample without dilution; B) day 8; sample without dilution; C) day 7, sample with dilution in 1000 µL of water.

Source: The authors
Figure 3 reogram between shear stress and shear rate for an emulsion of 10% astaxanthin oleoresin at different temperatures.
Table 1 presents the values obtained from the power law at a constant speed of 30 rpm. Where it can be observed that the values obtained for the apparent viscosity (η) varied between 1.55 ± 0.35 to 0.18 ± 0.02 Pa s for 5 and 30°C, respectively. However, between 5, 10 and 20°C it can be seen that there were statistically significant differences (p <0.05) for the η, not occurring between 20 and 30°C. The highest average values of the viscosity were presented at low temperatures of 5 and 10°C, in relation to those of 20 and 30°C, this can be explained due to the flocculation that the emulsion presents and that was inhibited in a certain way at high temperatures, a phenomenon that has been explained by [19].
Table 1 Rheological parameters of the law of power model. The values for the apparent viscosity (η) were obtained at 30 rpm. The consistency index (K) and the flow behavior index (n) are represented.

Data with different letters in the same column indicate significant difference (p<0.05) analyzed by Duncan's multiple range test.
Source: The authors.
Regarding the consistency index (K) values varied from 60.59 to 1.99 Pa sn and for the index of flow behavior (n) values varied from 0.59 to 1.02, for 5 and 30° C respectively, this is because the temperature reduces the value of the consistency index, but increases the value of the flow behavior index [20].
It should also be noted that the elaborated macroemulsion behaves like a shear thinning fluid from 5 to 20°C, since it has a range in the value of n between 0.59 to 0.94; while at 30°C it behaved like a dilatant fluid with a n value of 1.02, according to that described by [21] that for values of 𝑛<1.0 the fluids have a shear thinning behavior; while for 𝑛>1.0 they are presented as a shear thickening fluid. The change in the behavior of the fluid observed in the emulsion as the temperature increases has been related to the reduction of the consistency index (K) and the increase in the index of the flow behavior (𝑛) [20], which could cause a change in the behavior of the fluid, going from shear thinning to shear thickening. The temperature that best describes the rheological behavior is that of 10°C, since it presents better values of adjustment, very close to 1.0 for R2 and more tending to 0 for χ2, SSE and RSME.
3.3. Chromatic stability
Table 2 shows the color measurements in the CIEL*a*b* system with their respective standard deviations; Firstly, a statistically significant difference (p <0.05) of the chromatic coordinates L*, a* and b* between day 0 and 6 was observed, with the values of the day of elaboration being lower (L* = 8.07 ± 0.08, a* = 19.78 ± 0.19 and b* = 12.38 ± 0.28) to those reached on day 6 (L* = 7.12 ± 0.08, a* = 19.23 ± 0.22 and b* = 11.46 ± 0.18). All the chromatic coordinates, L*, a* and b*, although they had statistically significant differences (p <0.05) in the course of storage, except in some moments that this did not happen, were due to the very low values of the standard deviations, so it can be inferred that they are not so marked between day 0 and day 32.
In previous works [22], it is established for oil-in-water emulsions enriched with esterified astaxanthin, that the values of the chromatic coordinate L* increase with time, while a* and b* decrease, which would explain a decrease of astaxanthin; However, when the emulsion has an antioxidant, its values are stable over time. It has also been described that in emulsions the values for luminosity (L*) tend to increase as the radius of the micelle increases to a maximum of 100 nm, after which L* begins to decrease [23].
Fig. 4 shows the variations of C* and h° presented by the macroemulsion during the stability analysis. As for C*, it can be observed that it varied between 20.38 ± 0.14 to 23.33 ± 0.09, presenting a decrease in the color intensity as time increased. On the other hand, for the tone (Hue) the variation was between 27.27 ± 0.67 to 32.04 ± 0.79 decreasing its value as the time increased; however, for both coordinates a stability is observed, since the decrease is around 6 units at most. In other foods, such as emulsions, it has been established that for C* the values decrease as time passes [24-26], because it is losing saturation and tone, which can be seen in Fig. 4.

Source: The authors.
Figure 4 Stability of color in the time of the oleoresin astaxanthin macroemulsion.
Fig. 5 shows the color variation (ΔE) presented by the macroemulsion during a period of 32 days with respect to day 0. ΔE increased with time presenting an initial value of 0.84 ± 0.24 and a final value of 3.83 ± 0.22; however, the greatest color difference with respect to day 0 was observed on day 24 with a value of 4.19 ± 0.18. It is established that the macroemulsion did not present a color difference (ΔE) perceptible by the human eye, since a perceptible difference is considered when the value of ΔE> 5.0 [27].
Conclusion
The size of the micelles varied from 1.46 ± 0.73 to 2.31 ± 1.38 μm during the stability analysis, proving to be a macroemulsion in its size classification. In addition, a phenomenon was observed that accounted for a certain creaming and flocculation, which may be related to the movement of the micelles. The emulsion was presented as a shear thinning fluid, from 5 to 20°C, changing its behavior to shear thickening with T = 30°C. It should also be noted that the phenomenon of flocculation and coalescence directly affected the viscosity at low temperatures. The coloration of the macroemulsion tends to stabilize over time, not coming to be perceived by the human eye (ΔE> 5.0) during the storage period compared to day 0.