INTRODUCTION
Bacteria exhibit two modes of growth. Those that live free in a liquid medium, where they are dispersed, are called planktonic bacteria. When these planktonic bacteria are attached to a surface, they are called sessile cells. In the sessile state, these bacteria differ from their planktonic condition, because living in structured communities they begin to acquire some survival advantages [1]. Bacterial biofilms are formed when planktonic cells adhere to an inert surface and undergo several changes that culminate in the formation of an aggregate complex composed of sessile cells wrapped in an exopolymeric matrix. These complex structures are extremely resistant to the action of the immune system and antibiotics, being closely related to persistent infections. The most important biofilm-forming bacteria include Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa, leading to severe clinical complications often with lethal outcomes [2,3].
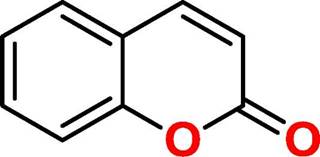
Coumarin (2H-1-Benzopyran-2-one, figure 1) is a natural product found in several plant species, which is related to a wide range of biological activities [4]. Among the applicabilities of coumarin, its antibacterial and antibiofilm potential has been shown [5-8], although its action is not completely clear. Based on the need for new therapeutic alternatives to treat infections caused by species producing biofilms, this study aimed to investigate the action of coumarin on planktonic and biofilm forms of S. aureus, K. pneumoniae and P. aeruginosa.
EXPERIMENTAL
Substances
The Coumarin (2H-1-Benzopyran-2-one) was obtained from Merck/Sigma-Aldrich* (Purity ≥ 98 %). The substance was properly weighed and solubilized in dimethyl sulfoxide (DMSO) at 5 % and Tween-80 at 2 %, to obtain emulsions in the concentrations necessary for use in the tests.
Strains
In this study, 15 bacterial strains were used: Staphylococcus aureus (ATCC-13150, LM-116, LM-182, LM-297, LM-356), Klebsiella pneumoniae (ATCC-700603, LM-143, LM-166, LM-176, LM-260), Pseudomonas aeruginosa (ATCC-15442, LM-163, LM-230, LM-286, LM-362). Except for the American Type Culture Collection strains (ATCC-13150, ATCC-700603, ATCC-15442), all other strains come from clinical isolates, which are part of the MICOTECA of the Laboratory for Research on Antibacterial and Antifungal Activities at the Federal University of Paraíba, Brazil. For use in the assays, bacterial suspensions were prepared in 0.9 % saline, from fresh cultures, and adjusted to the McFarland standard 0.5 scale.
Minimum Inhibitory Concentration (MIC)
Coumarin activity on planktonic cells was determined by the minimum inhibitory concentration (MIC) of the substance on the different strains. The analysis was carried out using the broth microdilution technique in a 96-well plate to obtain different concentrations of the substances [9]. (CLSI 2015). Initially, 100fiL of double strength Brain Heart Infusion (BHI) broth (Sigma-Aldrich/ Merck *) was added to the wells, and 100 μL of the substance emulsion was subsequently added. Serial microdilutions were performed in which a 100 μL aliquot from a well containing more concentrated medium was transferred to the next well with less concentrated medium, producing final coumarin concentrations ranging from 2048 to 4 μg/mL. Finally, 10 μL of bacterial inoculum was added to the wells such that each column contained a different strain. At the same time, sterility, cell viability and interference controls of vehicles used in the preparation of emulsions of substances (DMSO and Tween-80) were also performed. The test was performed in triplicate.
MIC is defined as the lowest concentration capable of causing complete inhibition of bacterial growth after 24 h at 35 ± 2 °C. The antibacterial activity of coumarin was interpreted and considered as active or inactive, according to the criteria already described in the scientific literature: up to 600 μg/mL, the substance is considered to have high activity, between 600 and 1500 μg/mL with moderate activity; and MIC above 1500 μg/mL characterizes the product with low activity or inactive against the tested strains [10,11].
Effect of coumarin on bacterial biofilms
The evaluation of coumarin activity on biofilm forms was performed using S. aureus, K. pneumoniae and P. aeruginosa strains. First, the potential of coumarin to inhibit biofilm formation was verified. For this, the microdilution plates were incubated statically with Brain Heart Infusion (BHI) broth containing different concentrations of coumarin in the presence of the bacterial inoculum (final concentration of 107 CFU/mL). Negative controls were carried out containing only culture broth and inoculum. After 24 h of incubation at 35 ± 2 °C, the contents of the wells were discarded and washed three times with sterile distilled water, to remove planktonic cells, reserving them for drying at room temperature. After drying, the 1 % violet crystal solutions were transferred and left to stand for 40 min. The dye was discarded and its excess on the well walls was removed by washing with distilled water. Thus, the wells received absolute ethanol and, after 30 min at rest, the plate was read in a microplate spectrophotometer (Thermo Scientific Multiskan GOV Waltham, Massachusetts, EUA) at 590 nm [12]. All analyzes were performed in quintuplicate.
Then, coumarin action on mature biofilms was verified. That is, the action on preformed biofilms. The plates were previously incubated with BHI broth and inoculum (final concentration 107 CFU/mL) for 24 h at 35 ± 2 °C, to form the biofilms in the wells. Then, the contents of the wells were removed and a new culture broth containing different concentrations of coumarin was added. The plates were incubated statically under the same conditions of time and temperature. Finally, the same process described above was performed for evaluation on a microplate spectrophotometer (Thermo Scientific Multiskan GOV Waltham, Massachusetts, EUA) [13].
Statistical significance was determined by the paired test using the t test and the results were considered statistically significant when p <0.05 for rejection of the null hypothesis. For this plot, the GraphPad Prism software (version 6.0 for Windows, San DIEGO, CA-USA) was used.
RESULTS AND DISCUSSION
Coumarin activity on planktonic cells was assessed by determining the minimum inhibitory concentration (MIC). The MIC range obtained was 256 to 1024 μg/mL (table 1).
Table 1 Minimum inhibitory concentration (MIC) of 2H-1-Benzopyran-2-one against bacterial strains.
| Strains | 2H-1-Benzopyran-2-one | Controls | ||
|---|---|---|---|---|
| MIC | Microorganism | Culture broth | ||
| S. aureus | ||||
| ATCC-13150 | 512 μg/mL | + | - | |
| LM-116 | 1024 μg/mL | + | - | |
| LM-182 | 1024 μg/mL | + | - | |
| LM-297 | 512 μg/mL | + | - | |
| LM-356 | 512 μg/mL | + | - | |
| K. pneumoniae | ||||
| ATCC-700603 | 256 μg/mL | + | - | |
| LM-143 | 512 μg/mL | + | - | |
| LM-166 | 256 μg/mL | + | - | |
| LM-176 | 256 μg/mL | + | - | |
| LM-260 | 512 μg/mL | |||
| P. aeruginosa | ||||
| ATCC-15442 | 512 μg/mL | + | - | |
| LM-163 | 512 μg/mL | |||
| LM-230 | 256 μg/mL | + | - | |
| LM-286 | 512 μg/mL | + | - | |
| LM-362 | 256 μg/mL | + | - | |
(+): bacterial growth; (-): absence of bacterial growth.
The MIC50 of coumarin was 512 μg/mL on the bacterial species used in this study, indicating moderate antibacterial activity, according to the classification criteria for substances of natural origin [10]. These values corroborate with other authors. Smyth et al. (2009) and Souza et al. (2005) observed coumarin action, with MIC ranging from 500 to 1000 μg/mL and better activity on Gram-negative strains, as well as what was identified in the present study (table 1). It is suggested that the antibacterial activity of coumarins and their synthetic derivatives is due to the inhibition of DNA gyrase pre-venting supercoiling, in addition to possibly also acting as photosensitizers for thymine dimers [14]. However, these mechanisms are not fully understood.
When evaluating the activity of coumarin on biofilm forms, a strong activity was noted in inhibiting the formation of biofilms by the three bacterial species studied (Figure 2A-C). The average biofilm inhibition was 89 % in MIC, 91 % and 93 % in 2x MIC and 4x MIC, respectively. Even at subinhibitory concentrations, coumarin was still able to significantly reduce the biofilm formation.

Figure 2 Effect of coumarin on biofilm forms. Inhibition of biofilm formation: A. S. aureus. B. K. pneumoniae. C. P. aeruginosa. Action on mature biofilms formed by D. S. aureus. E. K. pneumoniae. F. P. aeruginosa. Statistical analysis compared to the negative control (NC): **** p < 0.0001.
Biofilms are much more resistant to antibiotics and can tolerate concentrations 10 to 1000 higher than their respective planktonic forms. Several conventional antibacterial drugs are ineffective against biofilms, even in high concentrations [2]. Another major problem is that some drugs induce an increase in the formation of biofilms when they are in subinhibitory concentrations. During the infection treatment, part of the microorganism's population is exposed to suboptimal concentrations and low doses of these drugs can complicate these diseases [3]. Coumarin did not increase biofilm formation under these conditions, which makes it an interesting molecule to be investigated for this purpose.
Observing coumarin effect on mature biofilms, which, once established, are difficult to remove, there is a significant capacity to act on them, mainly in 4 x MIC (figure 2D-F), where there is an average reduction of 94 % compared to negative controls.
Lee et al. [ 7] and Zhang et al. [ 8] identified coumarin's strong potential to reduce E. coli and P. aeruginosa biofilms, respectively, also affecting other virulence factors, although the mechanism by which this occurs is not yet fully understood. In addition, other studies demonstrate that structural modifications based on the chemical skeleton of coumarin result in a great improvement in activity against planktonic and biofilm forms [15,16].
CONCLUSION
Based on this, it is concluded that coumarin has strong activity against planktonic and biofilm forms on the three species of great relevance in the clinical scenario. It is interesting that more studies investigate this potential of this natural product, to enable a pharmacological alternative for the treatment of these infections.