INTRODUCTION
Acinetobacter baumannii is a ubiquitous, non-glucose-fermenting, Gram-negative, oxidase negative, aerobic, pleomorphic, and non-motile coccobacillus (typically 1.0-1.5 µm by 1.5-2.5 µm in size) responsible for a significant proportion of healthcare-associated infections (HAIs) worldwide [1, 2]. The HAIs caused by A. baumannii mainly affect immunocompromised individuals, and are especially frequent in the Intensive Care Unit (ICU) acquired infections [1-4]. This pathogen has been associated with several types of infections, including pneumonia (mostly mechanical ventilator-associated), urinary tract infections, bacteremia, osteomyelitis, skin and soft tissue infections, and meningitis [5, 6]. Generally, these infections are caused by strains of A. baumannii that are resistant to the various antibiotics of choice, making pharmacological therapy a major challenge for healthcare professionals worldwide [6]. The presence of multi-drug resistance (MDR) is common among isolates of A. baumannii from hospitals, limiting treatment options and leading to increased morbidity and mortality of HAIs caused by this pathogen [3, 6].
Carbapenem antibiotics such as imipenem, meropenem, doripenem, but not ertapenem, are considered an important treatment option for MDR-A. baumannii infection. However, a considerable increase in the number of CRAB strains has been documented worldwide, inclusive in Brazil [7], and the extensive-drug resistance (XDR) phenotype stands out among the isolates of the A. baumannii. This critical panorama has stimulated the World Health Organization (WHO) to classify CRAB as a top priority organism for research and development of new antibiotics, with the aim to control the rapid and worrying advance of this bacterium in hospital environments [8, 9].
Resistance to carbapenems can occur through the combination of different mechanisms, such as decreased permeability of external membranes, alteration of the affinity of penicillin-binding proteins (PBPs), overexpression of efflux pumps, and production of carbapenem-hydrolyzing ß-lactamases (carbapenemases) [10]. The main mechanism of resistance to carbapenems between A. baumannii types is the overexpression of carbapenemases. Three types of carbapenemases have been recognized in this Gram-negative bacterium, namely Ambler class A ß-lactamases (i.g., GES-14 and KPC), Ambler class B metallo-ß-lactamases (e.g., IMP, VIM, SIM-1, and NDM) and Ambler class D oxacillinases (OXAs) [10]. Among the different carbapenemase types, the OXAs play an important role in the resistance of A. baumannii isolates to carbapenems, and has been reported in several countries as the main determinant ofresistance [11]. It has been described in different families in this species: intrinsic, chromosomally located and also found in plasmid OXA-51-likes (Neves et al., 2016); and the acquired OXA-23-like, OXA-40-like (formally OXA-24-like), OXA-58-like, OXA-143-like, and OXA-235-like [12, 13]. The presence of insertion sequences (IS) immediately upstream of bla OXA genes is known to induce the overexpression of OXA-51, OXA-23, or OXA-58, and to generate carbapenem resistance at a high level in A. baumannii.
The treatment of HAIs caused by CRAB is limited to the use of antimicrobials such as of polimixins or tigecycline [14]; however, it is known that the infusion of these drugs in monotherapy can favor the selection of strains with intermediate or complete resistance, especially to tigecycline [15, 16]. Associated with this, the emergence of a deter minant of colistin resistance carried by plasmids (i.e., the mcr gene) has been described [17]. Furthermore, the Eurofins Surveillance Network demonstrated that resistance to colistin among Acinetobacter spp. has more than doubled in recent years (2.8 % in 2006-2008 and 6.9 % in 2009-2012). Moreover, the polymyxins are characterized by a concentration-dependent activity and narrow therapeutic window, presenting with nephrotoxic and neurotoxic effects are well known. Thus, the poor therapeutic arsenal against CRAB, coupled with safety limitations and pharmacokinetic issues among polimixins, makes the epidemic of CRAB infections even more critical.
The emergence of resistance to carbapenems in A. baumannii is highly significant in emerging countries such as Brazil. Since the first description, the incidence and prevalence of CRAB have increased in this country, especially in last decade [18, 19]. However, despite the large number of reports of these infections, there are still no studies aiming to define the overall profile on infection rates and resistance of these isolates in Brazil, making it difficult to design national infection control measures to combat the spread of CRAB. Thus, this study aimed to evaluate, through a systematic review of the biomedical literature and meta-analysis of recovered data, the susceptibility profile to tigecycline and polymyxins of CRAB recovered over the last 10 years in Brazil. In addition, this study also describes the main mechanism of resistance to carbapenems amongst Brazilian CRAB isolates, and determines the frequency of each type of car-bapenemase, identified through the analysis of subgroups.
MATERIALS AND METHODS
A systematic review and meta-analysis was conducted according to the Cochrane Handbook guidelines [20]. In order to conduct the review, the steps of searching, selecting, extracting the data of interest, and analyzing results were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Prisma) statement [21].
Search strategies
A systematic search was conducted using PUBMED/MEDLINE, Scopus, Scientific Electronic Library Online (SciELO), Web of Science, Cochrane library, LILACS, and Virtual Health Library (VHL) databases, for articles published in English or Portuguese, using the following Medical Subject Heading (MeSH) terms and key words: ("Acinetobacter" OR "Acinetobacter baumannii" OR "Acinetobacter Infections" OR "Infections, Acinetobacter" OR "Acinetobacter Infection*") AND "Brazil". All details of the search strategy are showed in the supplementary file. Additionally, we screened the reference lists of all included studies and relevant systematic reviews, in order to identify additional eligible studies. The search was conducted up to October 18, 2019, and no date limits were established.
Inclusion and exclusion criteria
Articles reporting CRAB infections among hospitalized patients in Brazil were included using the PEOS strategy, as follows: "Population", bacterial isolates from patients of both sexes and all age ranges, recovered from patients hospitalized in Brazil; "Exposition", infection by CRAB; "Outcomes", resistance against polymyxins (i.e., polymyxin B and colistin), and tigecycline and carbapenemases type identified; "Study design", observational studies and hospital epidemiologic surveillance programs.
Studies were included on the conditions that: (i) clinical species such as A. baumannii were correctly isolated and characterized; (ii) the studies have evaluated a susceptibility pattern to polymyxins, using standard broth macro- or micro-dilution methods, according to the Clinical Laboratory Standard Institute guidelines (CLSI) or European Committee on Antimicrobial Susceptibility Testing (EUCAST); (iii) the article showed original material and was written in English or Portuguese; and (iv) it was published from 2009 onwards. Review articles, e-mails and editorials were excluded, as well as: (i) articles conducted in Brazil, but which used isolates from other countries; (ii) studies that did not show the identification method used for A. baumannii or the resistance mechanism; and (iii) articles that did not identify the genus/species of the isolates. In the event that the article complied with the inclusion criteria but the full text was not available, the corresponding author was contacted by e-mail up to three times (with intervals of 14 days), and the study was included if it was sent before the final contact.
Study selection
The titles, abstracts and keywords were first analyzed by two independent authors (A.P.C. and W.G.L.), in accordance with the inclusion criteria. Next, the pre-selected articles were subjected to a full-text evaluation, in order to decide whether to include or exclude the study. Ifthere was disagreement between authors, a third researcher (M.C.P.) was consulted by consensus, and the kappa coefficient (performed with 95% confidence interval) was used to analyze the degree of agreement between evaluators [22].
DATA ANALYSES
Articles that filled all inclusion criteria were submitted to an analytical full-text reading, and the following data were extracted: (i) reference (authorship and year); (ii) period and place (city and country) of data collection; (iii) type, origin and characteristics of samples studded; (iv) number of specimens collected; (v) the microorganisms identified and method employed; (vi) antibacterial resistance data ([CRAB], poly-myxin-resistant A. baumannii [PRAB], tigecycline-resistant A. baumannii [TRAB]); and (vii) molecular mechanisms of resistance. All data of interest were summarized in tables, for further critical analysis and interpretation by authors.
Statistical analyses
The prevalence of carbapenemases (OXAs, KPC and MBLs), and resistance to colistin and tigecycline between the CRAB reported in the included studies, were evaluated by meta-analyses. Resistant determinant references were grouped in Excel according to: study, year, isolated samples, and percentage of isolates according to each resistance mechanism. Next, the data were evaluated for meta-analysis according to random effect and confidence interval (95.0 % CI) through RStudio1 Software, using the meta package and metaprop command. The heterogeneity of the primary data was determined by the I-squared (I2) index, considering I2>50% as substantial heterogeneity. In order to calculate the frequency of carbapenemases, and resistance to colistin and tigecycline between CRAB recovered from hospitalized patients, either the fixed-effect model in low heterogeneity or the random effects model in large heterogeneity were used to pool data. Furthermore, a subgroup analysis was performed to evaluate the frequency of each carbapenemase type among CRAB isolates of Brazil.
RESULTS AND DISCUSSION
A.baumannii is a pathogen particularly important as a causative agent of HAIs. The emergence of resistance to carbapenems is notable in this bacterium, and the frequency of carbapenem-resistant A. baumannii (CRAB) is very high in emerging countries, such as Brazil [2, 4, 18]. These isolates typically exhibit resistance to other classes of antimicrobials, such as quinolones, aminoglycosides, and other ß-lactam compounds [19]. Thus, with few therapeutic options to treat infections by CRAB, the only option is the use of tigecycline or polymyxins. Here, we investigated the susceptibility profile to tigecycline and polymyxins of CRAB recovered over the last 10 years in Brazil, and described the main mechanisms of resistance to carbapenems that circulate among these isolates.
The search process in the databases resulted in 882 articles; 433 in Pubmed, 317 in Scopus, 6 in Cochrane Library, 95 in Virtual Health Library, and 31 in SciELO (figure 1). After identifying and excluding repeated articles between the databases, 69 studies were obtained and analyzed according to the eligibility criteria. From these articles, 48 were excluded, and the main exclusion moieties were studies that did not identify Acinetobacter at the species level, or included Acinetobacter non-baumannii (n=13); articles prior to 2009 (n=26); and studies designed without inclusion criteria as revision articles (n=6) (figure 1). Furthermore, two studies that did not include the resistance profile dates and one article which studied only isolates from colonized patients were also excluded from this review. Finally, 21 studies that met the eligibility criteria were selected for extraction of the variables of interest. The degree of agreement between two authors was considered substantial, according to kappa test (kappa=0.764).
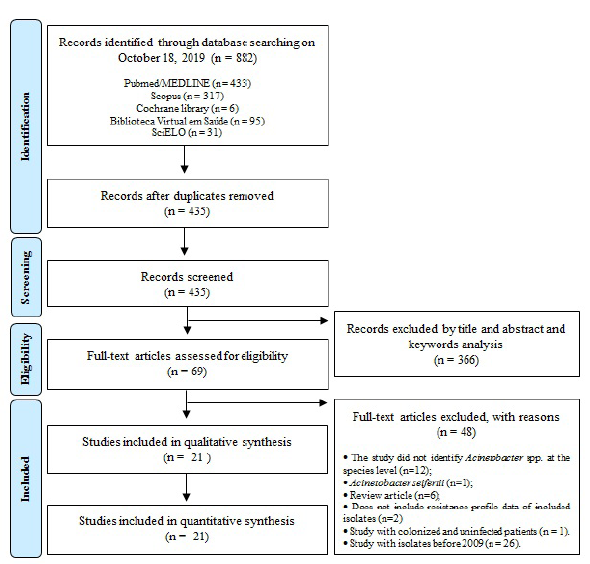
Figure 1 Flowchart of the selected studies for the systematic review according to the Prisma criteria.
Studies on carbapenem-resistant A. baumannii have been further explored in recent years around the world. For instance, the number of studies in PubMed reporting CRAB has increased from a single report in 2000 to over 266 reports in 2018, highlighting the global dissemination and good adaptation of this pathogen [23-43]. In the present study, most articles were published in 2016 and 2018. The increased interest in studying and investigating CRAB isolates, in response to the great clinical impact and advancing microbial identification techniques over the last five years (particularly with the introduction of MALDI-TOF/MS in clinical microbiology research centers), justifies the number of publications peaking in those years.
A total of 1,096 CRAB isolates, obtained from patients between 2009 and 2019, were analyzed in the selected studies (table 1). All included studies recovered one isolate per patient. Most studies were published in 2016 (6/21; 28.6 %) [11, 23-27] and 2018 (5/21; 23.8 %) [12, 13, 28-30] and the majority were conducted in the south region of the country (7/21; 33.3%) [12, 13, 23, 31-34], followed by the southeast (5/21; 23.8 %) [24, 27, 29, 31, 34], mid-west (3/21, 14.3 %) [30, 35, 36] and northeast (3/21, 14.3 %) [25, 26, 37]. Two studies involved different Brazilian regions (9.5 %) [38, 39]. The prospective design (9/21; 42.8 %) [13, 23, 25, 27, 29, 36, 37, 40, 41] was more common than retrospective (5/21; 23.8 %) [31, 32, 34, 38, 39]. Five case reports were also included (5/21; 23.8 %) [11, 24, 26, 30, 33].
Table 1 Main characteristics of included studies.
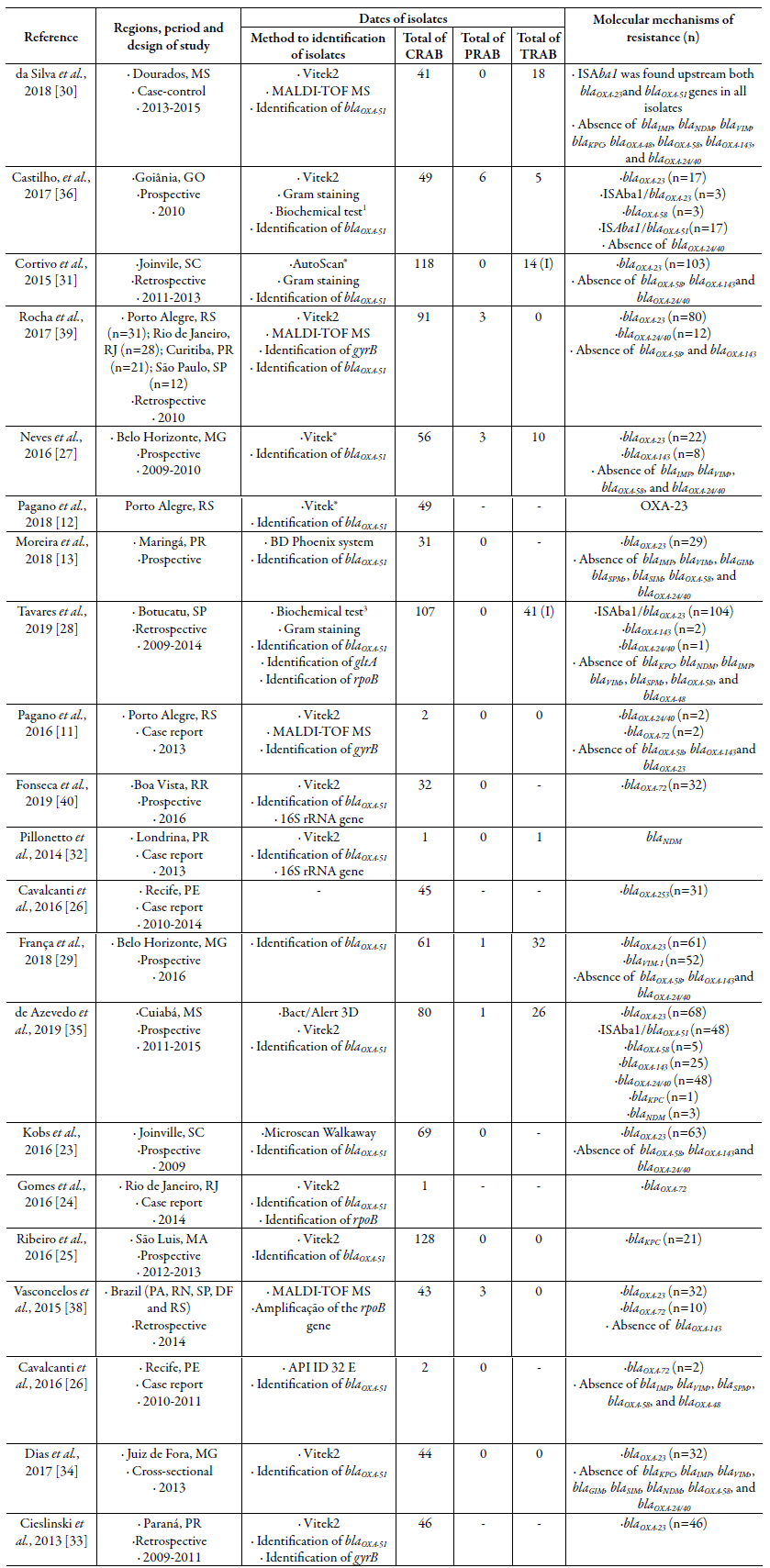
- :No identified or reported; IMP: Imipenem; MEP: Meropenem; POL: Polymyxin B; MALDI-TOF MS:.; OXA: Oxacilinase; KPC: Klebsiellapneumoniaecarbapenemase; NDM: New-delhi metallo-betalactamase.1Motility, growth at 42 oC, citrate utilization, oxidase and urease production, oxidative/fermentation, (OF)-glucose test, bile esculin hydrolysis test, decarboxylation of amino acids (i.e., lysine, ornithine and arginine) and OF-lactose test at 10%.2Oxidation/fermentation test activity, motility, oxidase production, catalase, glucose fermentation, urease activity and hemolysis of sheep blood.3Oxidase-negative, catalase-positive, glucose oxidation, ability to grow at 42 ° and 44 °C.
Regarding the states where the studies were conducted, the most frequent were Parana [13, 33, 34] and Minas Gerais [27, 29, 35], each with 3 studies (3/21; 14.3 %). The state of Paraná was where the first CRAB outbreak occurred in two different hospitals, making this a "model state" to study the resistance dynamics of this XDR bacterium in Brazil (Anvisa, 2013).
The most-used method for microbial identification was the detection of the gene bla 0XA - 51 (18/21; 85.7 %) [12, 13, 23-25, 27, 29-37, 39, 40], which is intrinsic in A. baumannii. The detection of bla 0XA-51-like can be used as a simple and reliable way of identifying A. baumannii, as this gene is found in virtually every isolate of this species [42-44]. However, the bla 0XA-51-like gene has also been found in other species from the Acinetobacter calcoaceticus-A. baumannii (Acb) complex, such as Acinetobacter nosoco-mialis [44].
Therefore, it is necessary to incorporate additional identification methods, and the combination of the automated VITEK-2® (bioMerieux, France) system with molecular biology (i.e., identification of gyrA, rpoB, or bla 0XA51-like gene) was the second-most used form of bacterial identification in the studies included in this review (15/21; 71.4 %) [12, 13, 23-25, 27, 30-35, 39, 40]. In contrast to the VITEK-2® system alone, which could not correctly identify A. baumannii and failed in predicting carbapenem susceptibility [45], the combination of VITEK-2® with genetic techniques increased significantly the performance of this identification method.
According to this study, the main determinant of resistance in CRAB isolated over the last decade in Brazil was the presence of OXA-type carbapenemases. The frequency calculated by the meta-analysis was 98 % [95 % CI: 0.91; 0.99; I2 = 95 %] (figure 2). During the meta-analysis, a high level of heterogeneity between the included studies, as shown by the I-squared (I2) index (I2 = 95 %), was observed, and thus all data were analyzed following the random effect model.
In fact, several other reviews point out that class D carbapenemases (OXAs) are the principal mechanism of resistance to carbapenem among isolates of A. baumannii [42, 46, 47]. Although all A. baumannii possess the bla OXA-51-like gene [48, 49], it is only expressed at levels compatible with carbapenem resistance after insertion sequence acquisition, specifically the ISAbal element [45, 50]. In this review, 62 % of isolates were reported to carry this element upstream of the bla OXA-51-like gene, revealing its importance among A. baumannii in Brazil.
Insertion sequences, also described as insertion sequence elements, are considered to be the smallest mobile DNA elements (not exceeding 2500 bp), and are very rapidly transferred between several microorganisms [51].
In addition, a subgroup analysis was performed to assess the most frequent OXA variant. As shown in table 2, the OXA-23 was the most frequent carbapenemase in CRABs from Brazil, showing a frequency of 91 % among the isolates included in this review [95% CI: 0.76; 0.97; I2= 97 %]. A similar rate was observed in South Africa, where >90% of A. baumannii isolates from HAIs were shown to produce OXA-23, while only 4% were found to have other types of carbapenemases (such as OXA-58) [52].
The blaOxA-23 gene is considered a virulence biomarker, and its presence in A. baumannii strains has been observed since 1985, when the first report was made in Scotland [46]. Since then, this gene has been spread to many hospitals worldwide, making it the most frequent carbapenemase found in A. baumannii [43]. In Brazil, the first report of the spread of OXA-23 appears to have started in Curitiba (Parana state) in 1999, when the first outbreak associated with CRAB-related infections occurred in Brazil [18]. Due to its importance, the Agência Nacional de Vigilância Sanitária [53] considers OXA-23 to be the main mechanism responsible for resistance to carbapenems in A. baumannii in country, which is in accordance with this meta-analysis.
The presence of metallo-beta-lactamase (MBL) in CRAB isolates recovered in Brazil between 2009 and 2019 was also analyzed. Here, 467 isolates were included, with 56 of them presenting some MBLtype. The meta-analysis revealed that, for this carbapenemase, a 12% frequency was found [95 % CI: 0.09; 0.15; I2=99 %] (figure 3), evidencing that MBLs are not frequently reported in A. baumannii isolates [54], these enzymes have recently started appearing in sporadic cases, in several parts of the world.
Currently, some MBLs have been reported in A. baumannii, such as imipenemase (IMP), German imipenemase (GIM),Verona imipenemase (VIM), Seoul imipene-mase (SIM), and São Paulo metallo-ß-lactamase (SPM) [55, 56]. In the analysis of carbapenemase subgroups (table 2), the VIM type (Verona Imipenemase) was the most common in Brazil, and a 15 % frequency was found [95 % CI: 0.12; 0.19; I2= 99 %] which is in concordance with isolates from patients admitted in ICUs in Iran, India, Saudi Arabia, and Korea [54, 56, 57].
Table 2 Frequency of different types of carbapenemases subgroup identified between carbapenem-resistant Acinetobacter baumannii recovered in Brazil (2009-2019).
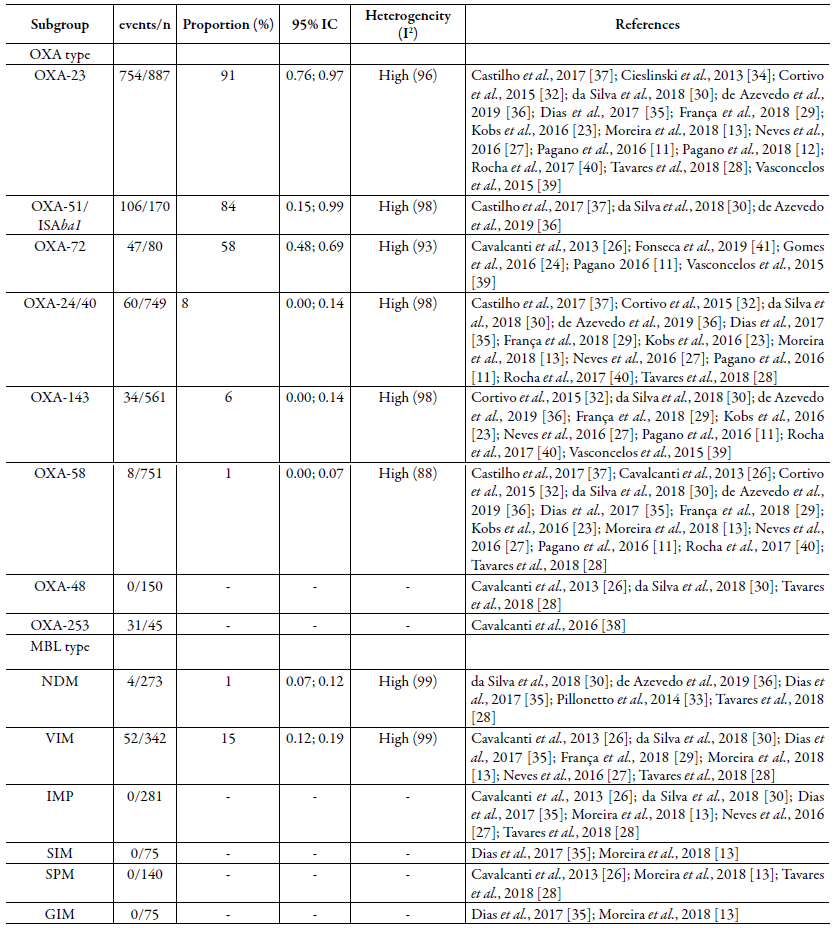
-: As only one study was dedicated to assessing the presence of this carbapenemases, subgroup analysis cannot be performed.
However, the presence of MBL in A. baumannii was lower in Brazil compared to Asian countries. For instance, a study from Iran which included 100 isolates of A. baumannii, collected from different clinical specimens of inpatients admitted to the largest teaching hospital in the north-west region of the country, showed that among 63 carbapenem (imipenem and meropenem) non-susceptible isolates, 31 (49 %) were found to be MBL producers. Of 31 MBL-producing isolates, 19 (61 %) carried the blaIMP gene and 9 (29%) carried the blaVIM gene [54].
The presence of New Delhi metallo-beta-lactamase (NDM) was also observed in only 1 % [95 % CI: 0.01; 0.04 I2= 99 %] of isolates, corroborating other studies from the United Arab Emirates and China, which also reported a low prevalence of this enzyme between A. baumannii isolates (1.2 % and 0.2 %, respectively) [56, 57]. However, Adler et al. (2018) [58] showed that, in an Israeli hospital, the prevalence of CRAB with the presence of the NDM enzyme was slightly higher (5.1 %), highlighting the regional differences in mechanisms of resistance to carbapenems circulating in A.baumannii.
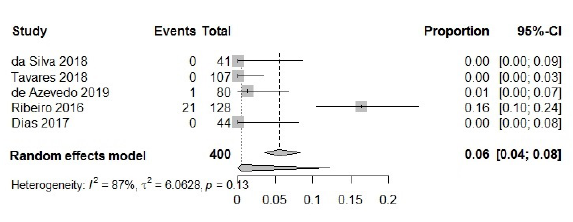
Finally, for Klebsiella pneumoniae carbapenemase (KPC), 400 isolates were included, in which 22 presented this type of resistance mechanism. The frequency calculated by the meta-analysis was 6 %, as reported by other authors [59, 60] [95 % CI: 0.04; 0.08; I2=87 %] (figure 4), and the random effect model was used. Subgroup analyses to variants of KPC were not performed, due to no studies being dedicated to typing this enzyme.
Although the estimated prevalence remains lower, since 2009 when it was first reported in Puerto Rico [44], this type of carbapenemase has increased in prevalence in recent years, with rates increasing from 3.4% to 14% in beta-lactam-resistant isolates [59]. In addition, this carbapenemase has already been associated with resistance to colistin and tigecycline for A. baumannii isolated in Portugal [60].
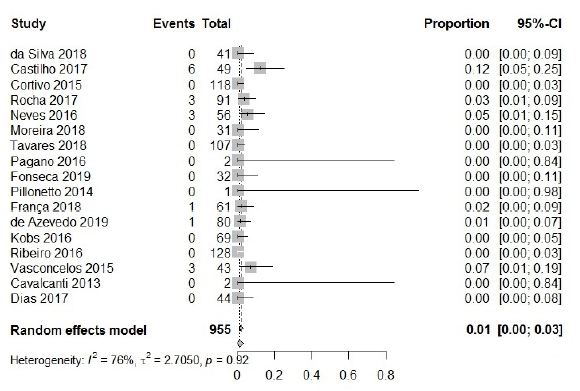
In the analysis of resistance to polymyxins, 955 isolates were included, with only 17 among them presenting resistance. The calculated frequency was 1 % [95 % CI: 0.00; 0.0, 3; I2=76 %], thus being antibiotic class with the highest activity against CRAB (figure 5). This observation is in accordance with reports from other countries, in which a low rate of resistance for colistin (2 %) and polymyxin B (3 %) was reported in different studies carried out in Iran [14, 61, 62]. These antibiotics are generally reliable agents against most A. baumannii isolates; however, resistance of A. baumannii to this class is emerging.
The use of colistin monotherapy has been associated with suboptimal clinical and microbiological outcomes. Furthermore, the A. baumannii hetero-resistance pheno-type is currently reported in much higher frequency than the resistance rate to car-bapenensin this bacterium, and the emergence of a determinant of colistin resistance carried by plasmids (i.e., the mcr gene) has already been described [17, 63, 64].
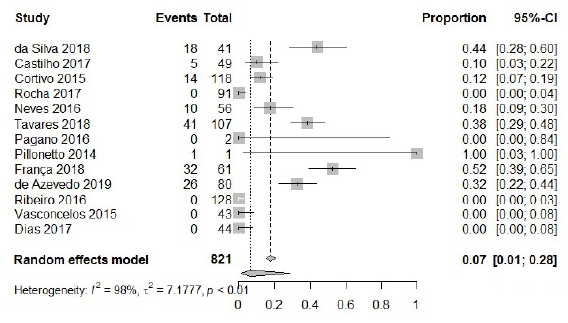
To analyze resistance to tigecycline between CRAB, 821 isolates were included. Among them, 147 presented intermediate or complete resistance to tigecycline, with a calculated frequency of 7 % [95 % CI: 0.01; 0.28; I2=98 %], and the random effect model was also used (figure 6). In Middle Eastern [66-68], south and southwest Asia [65-71] and Europe [72, 73], resistance to tigecycline can vary from 2-82 %, considering that Germany (6 %) [73] and Saudi Arabia (6.6 %) [65] have rates similar to those found in Brazil. In the Americas, non-susceptibility to tigecycline was found to be 5 % in North America [74], and between 0 - 20% in South America [75]. Thus, rates of non-susceptibility to tigecycline were lower in this review compared to other studies reported in Latin America.
Furthermore, four studies (4/21; 19.0%) demonstrated, concomitantly, isolates with resistance to carbapenems (CRAB), tigecycline (TRAB) and polymyxin (PRAB) [27, 29, 35, 36], characterizing a pan-drug resistance phenotype (PDR).
This review faces some limitations that should be considered when interpreting data. Data regarding characteristics of patients, antimicrobial use, and profile of health units are limited in the included articles, which the roles of these factors on the susceptibility profile of isolates. Also, the heterogeneity of the included articles was high. May be justified, samples from different origins were included in the studies (e.g., blood culture, sputum, urine, or wounds). Lastly, as data in most regions in the country were not available, our findings do not completely represent the profile of resistance of CRAB isolates from Brazil. In addition, due to the lack of identification of some carbapenemases in studies, relevant data may have been excluded.
CONCLUSIONS
In summary, we revealed that resistance to tigecycline in Brazil is lower, compared to in other countries in Latin America and in Asian countries. Regarding polymyxins, susceptibility remains high, and does not appear to be impacted by resistance to carbapenem in these isolates. In addition, oxacillinase is the main mechanism of resistance found in CRAB isolates in Brazil, with a major detachment for the presence of OXA-23, which is also considered an important virulence biomarker in this pathogen. Therefore, in order to prevent further dissemination of resistant isolates, appropriate diagnostic methods and infection control measures must be implemented. In addition, to prevent treatment failure, regular monitoring of antibiotic resistance by standard guidelines and methods is necessary, particularly for last-line antibiotics such as tigecycline and colistin.