INTRODUCTION
The risk of preterm delivery is defined as onset of labor generating changes in the cervix to allow for the descent and birth of the baby before week 381. Preterm delivery, defined as childbirth between 20 and 37 weeks plus 6 days of pregnancy, is the major cause of neonatal morbidity and mortality2. It is estimated that every year there are approximately 15 million preterm deliveries worldwide, which corresponds to 11.1% of all births3. A study led by the World Health Organization (WHO) found that the percentage of preterm deliveries in South America/Latin America is 8.1 and 7.9%, respectively, versus the total number of deliveries recorded in each region4. In Colombia, according to the figures of the National Statistics Department (Departamento Administrativo Nacional de Estadística - DANE), premature deliveries accounted for 20.1% of all births in 20165.
Risk factors associated with preterm delivery include maternal risks such as age (under 18 or over 40 years), low socioeconomic bracket, smoking,use of psychoactive substances or alcohol, excess physical activity, stress and malnutrition, uterine disorders, infections, a history of preterm delivery, rupture of membranes, multiple gestation, first and second trimester bleeds, and fetal causes, such abnormal placentation1,6.
It has been estimated that 28% of the fetal deaths that occur annually are due to preterm deliveries7. Neonatal morbidity and mortality are inversely proportional to gestational age at birth: 99% of preterm delivery-associated morbidity and mortality occur before 34 weeks2. Of babies born at 24 weeks, 80% will die, whilst 90% of the babies born during week 30 of gestation will survive. It has been shown that babies born at 22, 24 and 26 weeks of gestation show mortality rates of 54, 21 and 2% respectively, with higher disease-free one year survival rates greater than 0.02, 14.1 and 45.9%8. This means that prolonging pregnancy increases the probability of survival for the newborn.
Premature neonates have higher rates of neurodevelopmental disorders5, respiratory complications such as asthma and bronchitis9, and potential physical, psychological and economic consequences10. Long term impact on premature birth survivors include: visual impairment (blindness, myopia, retinopathy, hyperopia) in 25% of cases, hearing impairment in 5-10%, prematurity-related chronic pulmonary disease requiring oxygen supplementation at home (40%), cardiovascular disease including high blood pressure, reduced pulmonary function, higher asthma rates, growth failure and accelerated weight gain during adolescence. Neurodevelopmental problems include gait disorders, overall developmental delay, and psychiatric and behavioral sequelae (attention deficit/hyperactivity disorder, increased anxiety and depression disorder)10.
The diagnosis of preterm labor is based on the presence of regular uterine contractions causing cervical changes11. According to the Bogotá Health Secretariat clinical care guidelines, diagnosis is made in patients between 20 and 37 weeks of pregnancy, showing uterine activity of at least 4 contractions in 20 minutes, or 8 contractions in one hour, with intact membranes and cervical changes of 80% effacement and 2 cm dilation1.
Preterm delivery treatment is indicated in patients between 20 and 37 weeks, with regular uterine activity. Tocolytic treatment is contraindicated in patients rupture of membranes, chorioamnionitis, congenital malformations and fetal demise1. Treatment for preterm labor emphasizes hydration, since hypovolemia may be associated with increased uterine activity. However, tocolytic agents are used to inhibit uterine contractions with the purpose of delaying labor and achieving an effective maturation of the fetus1,2,12. There are different drug families that may be used as tocolytic agents, including β2-agonists, calcium channel blockers, oxytocin receptor antagonists, and cyclooxygenase inhibitors13. The choice of a tocolytic agent is based on the patient’s particular characteristics, and the drug’s safety profile and effectiveness14.
Atosiban is an oxytocin receptor antagonist, a tocolytic agent approved in 2007 by the National Food and Drug Surveillance Institute (Instituto Nacional de Vigilancia de Medicamentos y Alimentos - INVIMA) to delay imminent preterm delivery in pregnant women over the age of 18, with 24 to 33 complete weeks of pregnancy and normal fetal heart rate, presenting with threatened preterm delivery. INVIMA is the regulatory agency that issues approval for marketing medications in Colombia.
A review of meta-analyses found in the literature showed that none of the studies identified through database search assessed all outcomes or conditions of interest for our study, namely, maternal gestational age at the time of delivery, percentage of neonatal mortality, newborn respiratory distress syndrome, intraventricular bleeding, periventricular leukomalacia, necrotizing enterocolitis, percentage of maternal adverse events, and neonatal complications. Given the recognition that preterm delivery management is of paramount importance for reducing maternal and neonatal complications, this study focuses on evaluating the effectiveness and safety of atosiban versus nifedipine, indomethacin, terbutaline, fenoterol and placebo for the prevention of preterm delivery, taking perinatal and maternal outcomes into account.
MATERIALS AND METHODS
The final research question of this paper is shown in Table 1. This question was fine-tuned through expert consultation to define the need to limit the gestational age to that indicated by the National Food and Drug Surveillance Institute (INVIMA) (between 24 and 33 full weeks) and not consider magnesium sulphate as a comparator, as was initially suggested.
The inclusion criteria were the following:
Types of studies: Randomized clinical phase III trials with no publication date restriction, available in full text for comprehensive assessment when included in the review and meta-analysis.
Type of population: Studies that included adult pregnant patients with risk of imminent preterm delivery, defined as regular uterine contractions at least 30 seconds in duration and a frequency of more than 4 contractions every 30 minutes; cervical dilation of 1 to 3 cm (0 to 3 cm for nulliparous women) and effacement > 50%; gestational age 24 to 33 full weeks and with normal fetal heart rate (110-160 bpm) according to the expert panel and the Colombian guidelines for the management of preterm delivery1.
Type of intervention: The technology of interest was atosiban and the comparators were nifedipine, indomethacin, terbutaline, fenoterol, and placebo.
The primary effectiveness outcomes were absence of delivery at 48 hours and at 7 days, and secondary outcomes were the difference in gestational age at the time of delivery. Concerning safety, the primary outcomes were percentage of neonatal mortality and the proportion of maternal adverse events; the secondary outcomes were newborn respiratory distress syndrome, the frequency of intraventricular hemorrhage, the frequency of periventricular leukomalacia, and the percentage of total neonatal complications.
Any trials that were not available in full text but only as posters or abstracts were excluded because the complete information on the characteristics and outcomes of those references were not available for inclusion in the analysis.
Search Strategy
A literature search was conducted using the following databases: Medline via PubMed, EMBASE (Elsevier), Cochrane Database of Systematic Reviews (Wiley platform), Database of Abstracts of Reviews of Effects (DARE) (Wiley platform), Cochrane Central Register of Controlled Trials (CENTRAL) (Ovid platform), Lilacs (Virtual Heal Library - VHL, iAHx interface), WHO International Clinical Trials Registry Platform ICTRP portal, and ClinicalTrials.gov (Annex 1).
The key words used for the search were defined based on the PICOT question (Table 1). The first step was the inclusion of the terms to define the population and then the search terms for the technologies involved.
The criteria for defining the population as free text and controlled vocabulary (MeSH, Emtree and DeCS) were: “Obstetric labor,” “Premature” [Mesh] and “Preterm birth”. The terms for the health technologies of interest that were associated with the Boolean operator odds ratio (OR) were: “Nifedipine,” “Terbutaline,” “Atosiban,” “Indomethacin,” “Fenoterol” and “placebo”. Finally, the set of search terms that defined the population was combined with the terms of the health technologies of interest using the Boolean operator “AND”. The search terms used were adjusted according to the search platform of each electronic database. No filters were used for text availability (abstract), date of publication, type of study, or language (Annex 1).
Likewise, a manual “snowball” search was conducted based on the list of references of each article selected by the reviewers in search for other publications that met the previously defined search criteria.
Screening of references and selection of studies:
Prior to the start of the process, the selection criteria of the articles were shared and questions about the selection process were answered. The screening of references was conducted independently by two investigators (LS and PR), without knowing the results of the other. Afterwards, the articles selected by each reviewer were compared, any doubts regarding the selection of the articles were resolved by consensus between the reviewers, evaluating the new title and abstract, and in case additional information was required, the full text was obtained to finally make a decision of whether to include the articles or not. In case of disagreement, a third investigator was asked to participate (DR).
Assessment of the quality of the evidence The assessment of the quality of the evidence and the risk of bias was evaluated for each article in a paired manner by both investigators (LS and PR). The articles selected were evaluated using the tool designed by the Cochrane Collaboration for identifying any risk of bias15. This tool assesses the risk of the following biases: selection (random generation and blind assignment were taken into account); execution (blinding of the participants and the staff was evaluated); detection (outcome assessment risk was evaluated); attrition (the presence of incomplete data was assessed); and reporting (assessment for selective data reporting was performed). Based on these considerations, each article was categorized accordingly as: high, low or undetermined risk of bias. Disagreements were solved by a third researcher (DR).
Additionally, the tool developed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group was used to assess the quality of the evidence set found for each outcome16. This tool assesses the number of studies available for each outcome, study design, risk of bias, inconsistency in the results, the indirect nature of the results, inaccuracy, and other considerations (dose-response gradient and publication bias). Considerations for network meta-analysis assessment were taken into account17. Summary tables for quality assessment were reported in accordance with the proposed network meta-analysis model17.
Data extraction and evidence synthesis
For data extraction, the selected publications as well as the reports published as annexes and supplements were taken into consideration whenever it was necessary and depending on their availability. Extracted data included interventions, primary study inclusion and exclusion criteria, number of patients, age, clinical characteristics, type of analysis, outcomes assessed, ethical approval, site, and funding. Data for all the studies were uploaded to an Excel® worksheet. Data extraction was conducted by searching for the information reported as part of the intention-to-treat analysis. When available, information derived from safety analysis was considered, specifically in relation to adverse events. The total number of patients in each arm and the number of patients analyzed were considered for data extraction for the arms in each of the studies. Following data extraction, quality control of the information obtained was performed by means of comparison with the records of the primary studies. For adverse event analysis, all reported maternal events for each of the studies, both in the intervention group as well as in the control group, were added and compared.
Statistical analysis. After collecting the studies, assessments to determine head-to-head comparisons were performed. In those cases in which direct information was not derived from a comparison, the possibility of indirect comparisons using network meta-analyses was evaluated. To this end, the first thing that was determined was whether there was a comparison network that could enable an indirect comparison. When that was the case, the characteristics of the populations and the methods of each study were verified in order to assess transitivity within the information set found. In those cases in which this was not possible, a narrative report of the outcomes was made based on the data reported in the primary studies. If not, a network meta-analysis was conducted using the R statistical tool (R Development Core Team), version 3.2.3 and the R package netmeta, version 0.9-2, which uses a frequency analytical method. For categorical outcomes in each study, event and population numbers, or the comparison measurement, were extracted: risk ratio (RR), hazard ratio (HR), odds ratio (OR), with their respective scatter or confidence interval measurement for assessment15. For continuous outcomes, the mean for each group was extracted together with scatter or mean difference and confidence interval. A priori subgroup analyses were not considered.
This information was entered into the statistical software package using Microsoft Excel templates. Once the analysis was completed, the presence of I2 statistical heterogeneity was verified, categorizing it as suggested in the Cochrane manual18: not significant between 0 and 40%, moderate between 30 and 60%, substantial between 50 and 90%, and considerable between 75 and 100%. Additionally, the intra- and inter design Q test was performed in order to assess heterogeneity and consistency. Model consistency was verified by means of a comparison between direct available relationships and model estimates. The model contains estimates for all possible comparisons between all outcomes. Results were reported in league tables, showing the appropriate model estimates and direct results according to the outcome. Using a frequentist methodology similar to the Surface Undercumulative Ranking Curve (SUCRA), employed in Bayesian models and available in the netmeta package, the probability of being the best option among the ones used in the model was calculated.
Ethical considerations. Given that this research consists of a review of the literature and a meta-analysis, it is considered risk-free. Pursuant to Article 11 of Resolution 8430 of 199319, risk-free research is described as consisting of “studies that use retrospective document review techniques and methods, and studies where no intervention or intentional modification to the biological, physiological or social variables of the subjects is performed. These include clinical record reviews, interviews, questionnaires and other studies which do not identify or deal with sensitive subject behavior considerations”.
RESULTS
Overall, 5245 references were found as a result of the screening. After removing duplicates, a total of 4599 references were obtained. Of these, 30 which met the inclusion criteria by title and abstract were included for full-text evaluation. Finally, 11 studies20-30 corresponding to randomized clinical trials were selected for inclusion in the qualitative and quantitative analyses. The characteristics of the studies included are described in Annex 2; most were only two-arm studies. All the studies met the gestational age for inclusion in one of the following ways: consideration of the entire range of interest, partial consideration of the range of interest, consideration of a wider range, but reporting the information for the range of interest. The majority of the studies included a population of young women with a mean age ranging between 25 and 30 years. Singleton and twin pregnancies were considered in the majority of the studies. The reasons for reference exclusions are shown in Annex 3. Four records with no results were found in clinicaltrials.gov and 1 reference was a poster report and, for this reason, they were not considered in the results. For another study, there was a record stating that it had been withdrawn before the initiation of the trial. Of the remaining 9 studies, 9 did not represent the inclusion criteria for the populations (reports of gestational ages outside the range considered, excess cervical dilation), and two showed aggregate results for various comparators (beta-agonists together, medications and bedrest grouped together). Figure 1 shows the PRISMA reference screening flow diagram.
Risk of biases
The 11 phase III controlled clinical trials were assessed20-30. High risk of performance and detection biases was identified for 5 open-label studies20,21,23,24,28. For the remaining studies, low or indeterminate risk of selection, performance, detection, attrition reporting and other forms of biases were found. Risk-of-bias summary tables are shown in Figure 2.
Quality of the evidence
Annex 4 shows GRADE evidence profiles for important outcomes with evidence summary tables, together with the network geometry.
Effectiveness
Delivery delay of more than 48 hours. 8 relevant studies were identified for this outcome20,21,23-26,28,30 for a total of 1436 randomized patients; a network meta-analysis was performed. The results are summarized in Table 2. No statistically significant differences are found between atosiban and fenoterol (RR = 1.26; 95% CI: 1-1.59, moderate certainty), nifedipine (RR = 1.02; 95% CI: 0.91-1.15, moderate certainty) and terbutaline (RR = 1.06; 95% CI: 0.93-1.21, moderate certainty). Similar results are observed in head-to-head comparisons. No significant differences are found either between fenoterol, nifedipine and terbutaline (Table 2). In this analysis, I2 was 44.7% (moderate heterogeneity) with intra- and inter-design p value in the Q test of 0.11 and 0.24, respectively, that is, not statistically significant. No relevant inconsistencies were found for this analysis (Annex 4).
Table 2 Delivery delay longer than 48 hours*
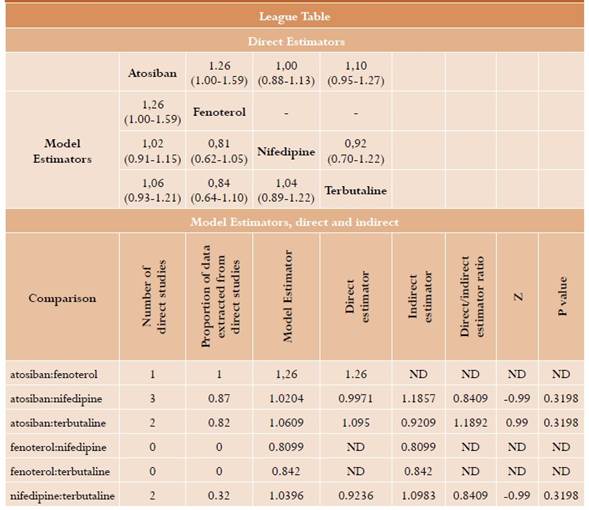
* We present the table with comparisons of each element reported in the column versus the element in every row. In the lower part are the model estimators; the direct tools are in the upper part, when available. The table including differences between direct and indirect estimators for each comparison is also reported with their respective statistical test.
Regarding the comparison between atosiban and placebo, the head-to-head study (22) reports a composite result together with the outcome of not needing additional tocolytic agents at 48 hours. For this comparison in women between 28 and 33 full weeks of gestation, the study found an absolute risk difference of 14% (95% CI: 4-23).
Delivery delay of more than 7 days: 7 relevant studies were identified for this comparison (20, 21, 23-26, 29) with a total of 1305 randomized patients; a network meta-analysis was performed (Table 3). No statistically significant differences were found between atosiban and fenoterol (RR = 1.18; 95% CI: 0.71-1.95, moderate certainty), nifedipine (RR = 1.06; 95% CI: 0.82-1.37, moderate certainty) and terbutaline (RR = 1.37; 95% CI: 0.99-1.89, low certainty). Similar results are observed in head-to head comparisons in all cases, except the comparison with terbutaline, where direct comparisons in 2 studies show a statistically significant difference. (RR = 1.61; 95% CI: 1.08-2.4) (Tabla 3). I2 for this analysis was 82% with good Q test intra- and interdesign p values of 0.0003 and 0.052, respectively. This corresponds to moderate to severe heterogeneity (Annex 4).
Table 3 Delivery delay longer than 7 days*

* We present the table with comparisons of each element reported in the column versus the element in every row. In the lower part are the model estimators; the direct tools are in the upper part, when available. The table including differences between direct and indirect estimators for each comparison is also reported with their respective statistical test.
As for the comparison with placebo, the head to-head study (22) provides a composite result together with the outcome of not requiring additional tocolytic agents at 7 days. For this comparison, an absolute risk difference of 17% (95% CI: 7-26%) was found in pregnant women between 28 and 33 full weeks of gestation.
Gestational age at the time of delivery. Four relevant studies were identified for this outcome (23, 24, 27 30), with a total of 849 randomized patients; a network meta-analysis was performed (Table 4). No statistically significant differences were found between atosiban and indomethacin (0.91; 95% CI: -7.74-5.92), nifedipine (-0,91; 95% CI: -3.54-1.71) and terbutaline (-0.13; 95% CI: -5-4.74). In this analysis, I2 was 0%, with an intra-design p value of 0.85 in the Q test, and undetermined inter-design value. There is consistency of direct and indirect comparisons in this case, given the network structure.
Table 4 Gestational age at the time of delivery*

* We present the table with comparisons of each element reported in the column versus the element in every row. In the lower part are the model estimators; the direct tools are in the upper part, when available. The table including differences between direct and indirect estimators for each comparison is also reported with their respective statistical test.
Safety
Neonatal mortality. Three relevant studies were identified for this outcome24,25,27. Based on the other studies with a total of 835 patients randomized, a network meta-analysis was performed (Table 5). A statistically significant difference was found between atosiban and indomethacin (RR = 0.21; 95% CI: 0.05-0.92, low certainty), but no significant differences were found with nifedipine (RR = 0.45; 95% CI: 0.19-1.1, low certainty) or terbutaline (RR = 0.5; 95% CI: 0.13-1.91, low certainty). No significant differences were found between indomethacin, nifedipine and terbutaline in the meta-analysis. Similar results are observed in the head-to-head comparisons (Table 5)24,25,27. No significant differences were found between fenoterol, nifedipine and terbutaline. Given the structure of the evidence, I2 and the Q test could not be calculated (see Annex 4).
Table 5 Neonatal mortality*

* We present the table with comparisons of each element reported in the column versus the element in every row. In the lower part are the model estimators; the direct tools are in the upper part, when available. The table including differences between direct and indirect estimators for each comparison is also reported with their respective statistical test.
Proportion of maternal adverse events. Five relevant studies were identified for this outcome20,21,23,26,28, for a total of 588 randomized patients; a network meta-analysis was performed (Table 6). Statistically significant differences were found between atosiban and fenoterol (RR = 0.16; 95% CI: 0.08-0.31, moderate certainty), nifedipine (RR = 0.48; 95% CI: 0.3-0.78, moderate certainty) and terbutaline (RR = 0.44; 95% CI: 0.28-0.71, moderate certainty). Similar results were observed in head-to-head comparisons, except the direct comparison between atosiban and terbutaline (RR = 0.55; 95% CI: 0.3-1.00), where point estimation is similar but the confidence interval does not show statistical significance. In this analysis, I2 is 0%, and the intra- and inter-design p values for the Q test are 0.61 and 0.24, respectively.
Table 6 Maternal adverse events*

* We present the table with comparisons of each element reported in the column versus the element in every row. In the lower part are the model estimators; the direct tools are in the upper part, when available. The table including differences between direct and indirect estimators for each comparison is also reported with their respective statistical test.
The evidence summary table for the network meta-analysis of this outcome is shown in Annex 4. Given that aggregate adverse events are considered in this analysis, the broken down information reported in the primary studies is shown below.
Table 7 shows the specific adverse events reported in each study against atosiban, together with the reported statistical analysis. There is evidence of a statistically significant reduction between atosiban and nifedipine in terms of hypotension and overall events23, a statistically significant reduction between atosiban and terbutaline in terms of tachycardia, tachypnea and dyspnea, and a statistically significant increase between atosiban and terbutaline in terms of nausea, vertigo and hot flashes26. No reported statistical tests were found for the other outcomes.
For the remaining safety outcomes (neonatal respiratory distress syndrome, intraventricular hemorrhage, intraventricular leukomalacia and neonatal complications) a report of the information presented in the primary articles was prepared, considering that there was insufficient information for a network meta-analysis or a simple direct metaanalysis (Table 8). There are no statistically significant differences between atosiban and nifedipine in the four events evaluated24. No statistical analysis is reported for the comparison with terbutaline, although a lower frequency of neonatal respiratory distress, intraventricular hemorrhage25, and total events26 is reported.
DISCUSSION
This study is a systematic review of the literature comparing atosiban versus other treatments in patients between 24 and 33 completed weeks of gestation. As far as effectiveness is concerned, no statistically significant differences were found when compared with nifedipine, terbutaline and fenoterol in terms of no delivery at 48 hours and at 7 days. These results are of moderate-to-low certainty in terms of evidence. When compared to placebo, one study reported significant differences in terms of the composite outcome of no delivery or need for additional tocolytic agents at 48 hours and at 7 days.
Concerning safety, lower neonatal mortality was found for atosiban when compared to indomethacin, and non-significant differences were found with nifedipine and terbutaline (low certainty). It is worth noting that it was possible to analyze this result from a network with a total of three primary studies. In terms of maternal adverse events, a probably lower frequency was found when compared to fenoterol, nifedipine and terbutaline (moderate certainty). It is worth highlighting that a combined analysis was performed for this outcome, probably combining adverse events of different nature. Consequently, it is reasonable to verify all the comparisons for each event in order to arrive at a more adequate personalization of the safety profile according to each individual patient. No significant differences were found in terms of neonatal respiratory distress, intraventricular hemorrhage, periventricular leukomalacia and neonatal complications.
How complete was the review in terms of the information obtained in terms of outcomes? Wide screening of the literature is designed to identify all the relevant data available in manuscripts published in journals or registry reports. It may be argued that the limitation in terms of gestational age between 24 and 33 full weeks may have limited the scope of the conclusions of this research, given that it meant that some studies were not included. However, we believe that this makes this review relevant for the local context, given that it focuses on the literature pertaining to the population for which atosiban is indicated in our setting.
Quality of the body of evidence. As reported in the evidence summary tables, the results showed medium-to-low accuracy. Regarding effectiveness outcomes, there are difficulties with the accuracy of the results in the report. They do not allow to differentiate clearly between the absence of a difference and lack of statistical precision for results without mathematical significance. For such outcomes it is not considered that within the network there were some open studies.
Regarding the body of evidence available for neonatal mortality, certainty is low. This occurs because of problems related to the accuracy of the tools used for estimation and the structure of the network, which implies that some comparisons come exclusively from first degree loops. For maternal adverse events, the body of evidence is also limited to moderate certainty due to the risk of bias of some open studies that may be relevant both for detection and performance.
Applicability of the results based on the quality of the evidence. Considering all of the above, it may be said that there are no important differences in effectiveness when compared to other active references (moderate certainty). In terms of the results on neonatal mortality, even though they may show interesting information, it is likely that more and better primary evidence is needed to consider them directly for decision making. However, these results should not be ignored. Results on maternal adverse events show a moderate certainty. There is a decrease in such events when compared to other active comparators. This may have implications for clinical practice. A closer look at reported events (Table 7) clearly shows that the decrease in atosiban-related adverse events is cardiovascular, such as hypotension, tachycardia and the like. This is a potentially important aspect since there are cases in which severe hypotension in the mother is associated with an increase in neonatal morbidity and mortality due to the decrease in placental perfusion2,31. This could imply an assessment by the clinician in order to determine whether the differences in these events may favor atosiban instead of other options. A similar rationale can be used for other reported adverse events.
The main strength of this study is the use of a systematic review methodology for searching and synthesizing available evidence regarding a specific question. The fact that the review considers randomized clinical trials adds to the strength of the body of evidence, because these optimize bias control for intervention questions. The extensive search in medical literature, amplified through the “snowball”, attempts to capture all relevant publications even though it cannot ensure absolute certainty. The use of evidence summary tables facilitates communication of results to clinicians. When reviewing meta-analyses available in the literature, none of those obtained in the database search assessed the full amount of the outcomes, or comparison markers of interest for our studies, for which a paper with incremental input to the existing literature could be considered. The population restriction to pregnancies between 24 and 33 complete weeks as indicated by INVIMA may help make the review more relevant from the local perspective.
Regarding its weaknesses, it is worth mentioning that the use of network meta-analyses may create weaknesses in the results, given that indirect evidence is of lower quality than direct evidence, broadly speaking. In this specific case, this effect is not necessarily large, given that in nearly all comparisons there was an important percentage of information coming from direct evidence. In addition, the network structure for some outcomes, such as neonatal mortality, implies many comparisons identical to those in direct studies, while indirect data are used only for notifying some outcomes which had no indirect comparison. The fact that Colombian populations are not included in the trials is a potential, though unavoidable limitation, and it possibly has a marginal effect on the results. The restriction of the population to pregnancies between 24 and 33 complete weeks could be considered as a limitation to the inclusion of relevant studies and generalizing results. This may translate into differences with other previous studies. For example, some results differ from those obtained in the meta-analysis carried out by Flenady et al.32, in which it was observed that there is no evidence suggesting atosiban is superior in terms of extending pregnancy when compared to placebo(32). The difference between the Flenady et al. meta-analysis and this trial may be due to the fact that this metaanalysis included two investigations in patients with gestational age outside the range of interest for the present study and outside the one set forth in the health registry. In this case, the information from the composite outcome resulting exclusively from a primary trial was included, which could limit the utility of this conclusion. However, it is useful because it sheds light on a potentially favorable effect of atosiban over placebo.
CONCLUSIONS
Atosiban probably shows no differences in effectiveness when compared to nifedipine, terbutaline and fenoterol in terms of labor delay at 48 hours and 7 days in pregnant women with risk of preterm labor between 24 and 33 weeks (moderate to low certainty). The comparison of atosiban against placebo based on a primary study, showing a possible improved performance of atosiban in terms of a composite outcome of labor delay and non use of tocolysis at 48 hours and at 7 days.
Regarding safety, there is possibly a lower frequency of events with atosiban when compared to indomethacin, whereas no statistically significant differences were found with nifedipine and terbutaline (low certainty).
Concerning maternal adverse events, potential reduction of events was found with atosiban compared to fenoterol, nifedipine and terbutaline. It is worth looking closely at the differences regarding specific adverse events versus each comparator to assess whether these have an impact on clinical behavior.











 text in
text in 










