Introduction
In Colombia, the Sistema de Gestión de Seguridad y Salud en el Trabajo (Occupational Health and Safety Management System) considers formaldehyde (FA) as a priority substance 1 since it is included in the list of carcinogens of interest to the Sistema de Vigilancia del Cáncer Ocupacional (Occupational Cancer Surveillance System)2 developed by the Instituto Nacional de Can-cerología (National Cancer Institute.)3
Even though the Occupational Diseases List published by the Ministry of Labor4,5 includes some of the pathologies associated with exposure to FA -such as acute bronchitis caused by chemical agents, pulmonary edema caused by chemical agents, inflammation of the upper respiratory tract caused by chemical agents, chronic diffuse emphysema, reactive airways dysfunction syndrome, pulmonary fibrosis, obliterative bronchiolitis and toxic effects-, diseases related to the carcinogenic potential of this substance have not been considered.
FA is a volatile organic compound with a characteristic and irritating odor, characterized by having a double bond with oxygen (H2C=O), which promotes its reactivity. FA is used dissolved in water at a maximum concentration of 40% and is produced on a large scale worldwide. An estimated 21 million tons per year6 of this compound are used to manufacture a large number of industrial products such as urea and melamine phenolic resins, which have various applications in adhesives and binders; wood products such as cellulose pulp for making paper; plastic products; paints for coatings; and products for the textile industry.7 In other areas, including the clinical field, it is used directly in aqueous solution as a disinfectant, tissue preservative, and biocide.
The main route of exposure to FA is inhalation8 and, depending on its concentration, exposure to this compound can cause different symptoms. At concentrations of 0.1-5ppm, it can cause eye irritation, tearing, upper respiratory tract irritation and coughing; at concentrations of 5-30ppm, it can cause chest pain, airway irritation, respiratory distress, headache, asthmatic reactions and can aggravate pre-existing respiratory conditions;9-11 and at concentrations of 50-100ppm, it can cause pneumonia, pulmonary edema and even death.12,13 Permanent exposure to lower concentrations of FA can produce nasopharyngeal and squamous cell carcinoma in the tissues of the nose.7
The International Agency for Research on Cancer14 classifies FA in the group of agents that are carcinogenic to humans (Group 1). Recent meta-analyses have reported a strong association between exposure to this substance and acute myeloid leukemia,15 while other studies with limited evidence have established a link between FA and sinus cancer.16-18
Other consequences of FA exposure have been reported. For example, Lino et al.19 found that this substance produces alterations in the physiological balance between oxidative and antioxidant enzymes in lung tissue, most likely favoring the oxidative pathway and generating lung inflammation. Schwensen et al.20 explained that skin irritation occurs after having contact with this substance and that this, in turn, can produce contact dermatitis. Thrasher et al.21 reported an association between recurrent exposure to this substance and immune system disorders since, in humans, FA conjugates with human serum albumin, forming a new antigenic determinant (F-HSA); this in turn causes the development of anti F-HSA antibodies. Finally, Thrasher et al.22 described that exposure to FA produces genotoxic and cytotoxic effects such as increased chromosomal aberrations, sister chromatids exchange, and presence of micronuclei.
Since FA is a compound widely used at industrial level, multiple research works have been developed using genotoxicity tests to identify the risks that occupational exposure to this chemical poses to the health of workers, showing possible damage to DNA. The objective of this study is to review recent research on occupational exposure to FA to design a strategy for monitoring and surveillance of workers occupationally exposed to this substance.
Materials and methods
A literature review was conducted in PubMed, MedLine, ScienceDirect, and Embase looking for human studies published between 2013 and 2017 in English or Spanish. The following descriptors were used: "occupational exposure", "formaldehyde", "mutagenicity test" and "DNA adducts", their Spanish equivalents and their combinations ("occupational exposure AND formaldehyde AND mutagenicity test OR mutagenicity test AND DNA adducts"). This search retrieved 103 articles whose titles and abstracts were analyzed.
Of the articles available in full text, those that met the following inclusion criteria were selected: assessed only exposure to FA, were conducted in occupational settings, and had quantitative results of airborne FA concentrations or genotoxicity tests that report the analytical technique used. In-vitro studies were excluded. Moreover, legal and technical sources were consulted to search publications that frame and regulate the monitoring of occupationally exposed workers (OEW) in Colombia, which should address the following issues: occupational exposure, medical monitoring, environmental concentration limits, FA and occupational cancer. Figure 1 shows the search flowchart.
Results
The 36 articles that met the inclusion and exclusion criteria were included. Most of the papers were observational studies in which the concentration of FA was quantified, and genotoxicity tests were performed to establish the relationship between exposure to FA and the health consequences among OEW. Some of the health effects reported in the articles include acute responses such as airway and eye irritation, while chronic effects were analyzed by means of genotoxicity biomarkers,23 which allow identifying and characterizing the damages that can be caused by this pollutant. Table 1 presents the results of the included articles that were considered most relevant to the objective of this study.
Table 1 Formaldehyde concentration and genotoxicity test in occupationally exposed workers.
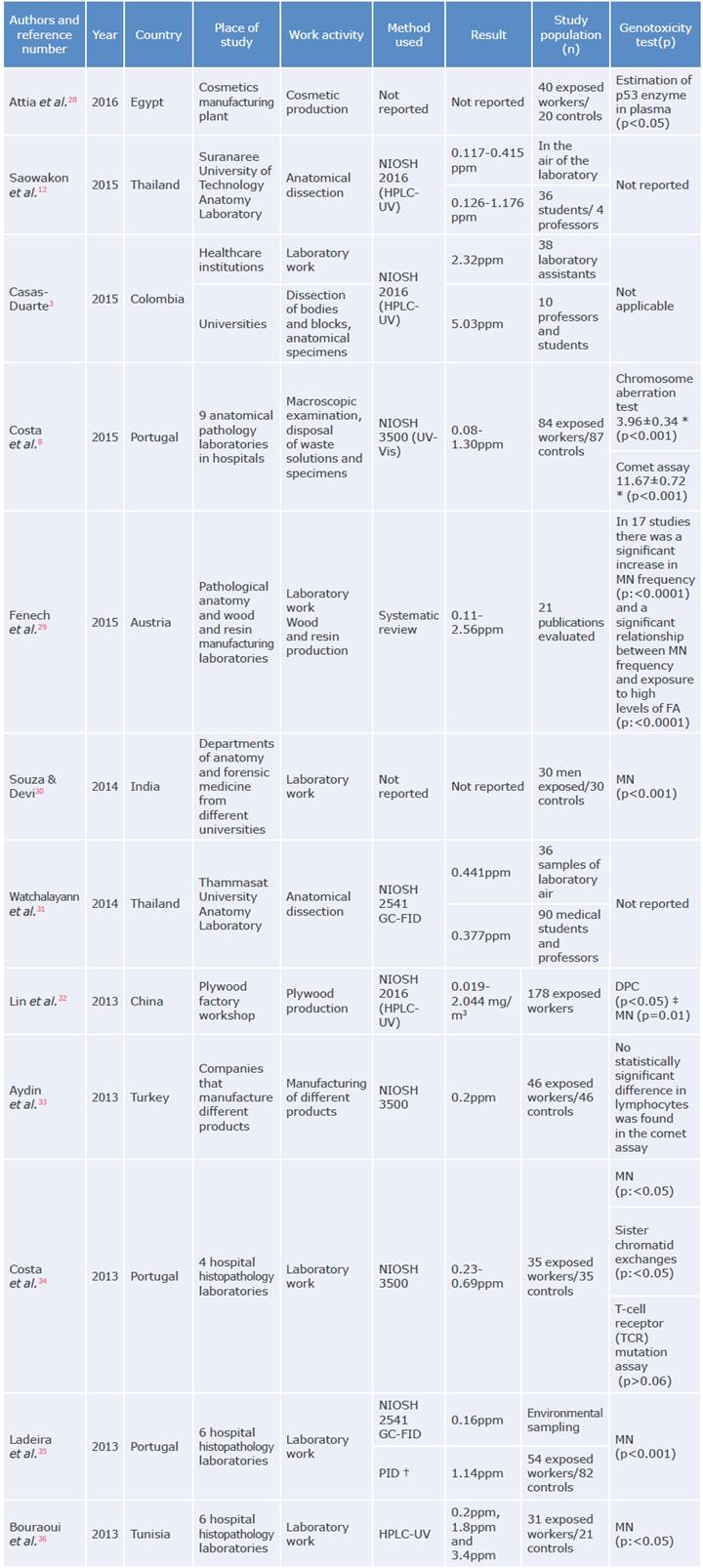
NIOSH: National Institute for Occupational Safety and Health; MN: micronucleus; OSHA: Occupational Safety and Health
Administration; HPLC-UV: high-performance liquid chromatography-ultraviolet; UV-Vis: ultraviolet-visible spectroscopy;
FA: formaldehyde; GC-FID: gas chromatography - flame ionization detector; PID: photoionization detector.* Mean±SD.
† Photoionization detector (11.7 eV lamp) with simultaneous video recording.
* Chromosome damage and DNA-protein cross-links in peripheral blood lymphocytes.
Source: Own elaboration.
The studies included in this review were carried out in Europe (38%), Asia (31%), South America (19%) and Africa (12%) in different working sectors, including the practice and teaching of health sciences, industrial manufacturing processes, and cosmetics production. The concern generated worldwide by occupational exposure to FA is evident in the increase in research on the subject in different work environments.
Discussion
Formaldehyde concentrations in working environments
According to the criteria of the United Nations' Globally Harmonized System of Classification and Labeling of Chemicals,37 any solution containing a carcinogenic substance beyond a concentration of 0.1% should be considered carcinogenic; however, 5% of FA solutions are used for cadaver dissection. Saowakon et al.12 reported concentrations of FA above permissible limits in both the air and the breathing zone of anatomy laboratory workers.
The concentration of FA can be quantified through standardized methods using different analytical techniques proposed by entities such as the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health. Table 2 depicts the methods used in the articles included in the review.
Table 2 Methods for quantifying formaldehyde in work environments.
| Agency | Method | Analytical technique | |
|---|---|---|---|
| NIOSH | 3500 | UV-Vis | UV-visible spectrophotometry |
| NIOSH | 2541 | GC-FID | Gas chromatography - flame ionization detector |
| OSHA | 1007 | HPLC | High-performance liquid chromatography |
| OSHA | 52 | GC-NPD | Gas chromatography -Nitrogen phosphorous detector |
| NIOSH | 2016 | HPLC-UV | High-performance liquid chromatography-UV detection |
| NIOSH | 3800 | FTIR | Fourier transform infrared spectroscopy |
NIOSH: National Institute for Occupational Safety and Health; OSHA: Occupational Safety and Health Administration.
Source: Own elaboration based on Kennedy,38 Occupational Safety and Health Administration39 and Kennedy & Williams40.
The highest concentrations of FA were found in studies conducted in China,32 Colombia3 and Tunisia,36 while the lowest concentrations, with values below permissible exposure limits, were found in a research conducted at a wood manufacturing center in Brazil.26 Studies by Lin et al.32 and Ghasemkhani et al.41 in China and Iran, respectively, found statistically significant differences in FA concentrations in the breathing zone of workers with the same job position depending on shift distribution and task performed.
The studies by Saowakon et al.12 and Ladeira et al.35, conducted in anatomical dissection laboratories, reported that the environmental concentration of FA in the breathing area of students and instructors was statistically different from the concentration found by fixed measurements in that area, the latter being higher.
In the articles included, the lack of engineering control systems, such as ventilation systems, extraction booths, localized extraction systems, among others, was identified. These systems would allow minimizing the concentration of FA in work environments.
Concerning medical surveillance, in 2015, Peteffi et al.42 conducted a study on workers of the furniture manufacturing industry in Brazil who were exposed to different levels of FA. The authors found that the levels of formic acid in urine were significant only in workers exposed to high concentrations of this compound.
In a study conducted at the University of Erlangen-Nuremberg, Schmid et al.43 compared the levels of formic acid in urine of 70 people not occupationally exposed to FA to the levels of 30 medical students attending anatomy classes during their practice with high exposure levels for a short period and of 8 pathology laboratory workers with long exposure periods. FA concentrations in the group of students were 0.32-3.48ppm and the levels of formic acid in urine fluctuated, so they were associated with the diet and not with the amount of FA in the air (p=0.070). In the group of workers, there was also no linear correlation between the levels of formic acid in urine and the concentrations of this pollutant in the air.
In 2010, Mautempo et al.44 conducted a study on 31 workers, in whom they found significantly elevated levels (p<0.0001) of formic acid in urine compared to the control group. However, the results do not describe the environmental concentration of FA, so no relationship can be established between concentrations of formic acid in urine and exposure to the pollutant.
Genotoxicity testing in workers occupationally exposed to formaldehyde
In two studies conducted in 2016 by Peteffi et al.26 in a furniture factory and Peteffi et al.27 in beauty salons, no significant differences were observed in the micronucleus test between OEW and the control group, while the comet assay showed significant differences, even though the workers were exposed to low concentrations of FA. These results are similar to those described by Zendehdel et al.,24 who observed that DNA damage in peripheral blood lymphocytes can occur even in controlled work environments. For their part, Ladeira et al.45 reported an increase in the frequency of micronuclei in exfoliated buccal cells and in peripheral blood lymphocytes in OEW and found significant differences compared to the control group.
Regarding the use of genotoxicity biomarkers, in a study carried out on workers in the cosmetics manufacturing industry, Attia et al.28 proposed that malondialdehyde (MDA), a metabolite and reactive oxygen species resulting from lipid peroxidation, and the mutation of the p53 gene, a known indicator of carcinogenesis, could be considered biomarkers of genotoxicity, as they found statistically significant differences in these two biomarkers between the OEW and the control group.
In an investigation conducted on 84 workers from the pathological anatomy service of different hospitals in Portugal, who were exposed to FA at levels higher than those allowed, Costa et al.8 found chromosomal aberrations and aneuploidies in the population studied through a structural chromosomal aberration test and a comet assay.
Furthermore, two systematic reviews were included, one by Fenech et al. ,29 who found significant differences in micronucleus frequency between workers exposed to high concentrations of FA and the control group in 17 of the 21 included studies, and another by Chiarella et al.,46 who proposed protein adducts as a potential biomarker after reviewing 95 studies.
Toxicological aspects of formaldehyde
Toxicokinetics
FA is produced endogenously in small quantities as part of the human body's metabolism. Its blood concentration reaches about 1.5-3 mg/L and is generated in processes such as methylamine deamination, methanol oxidation and histone demethylation by cytosolic alcohol dehydrogenase.14
When individuals are exposed to FA exogenously, this compound is absorbed by inhalation or ingestion. Therefore, the upper respiratory tract is its main route of entry into the human body and the nasal and nasopharyngeal mucosa are its target tissues; it has not been found significantly in other organs. Figure 2 shows the metabolic pathways that take place after entering the body.47 FA can be converted to methanol by the alcohol dehydrogenase-1 (ADH1) enzyme or oxidized to formate. Mitochondrial oxidation is catalyzed by the formaldehyde or aldehyde dehydrogenase-2 (ALDH2) enzyme, while cytoplasmic oxidation is catalyzed by the alcohol dehydrogenase-3 (ADH3) enzyme to form S-formylglutathione and then formate.46 Once formate is incorporated into the metabolic pathways, it can continue to oxidize towards carbon dioxide; another secondary metabolic pathway dependent on the tetrahydrofolate cofactor has also been reported.46 Moreover, FA acts by creating reversible or irreversible adducts with macromolecules(RNA, RNAand proteins), which leads to mutations and proliferation of micronuclei in the cells.14 MacAllister et al.48 evaluated the excretion pathways of this pollutant in animal models and concluded that the main pathway is exhalation (40%), followed by urine (17%), and feces (4%).
Toxicodynamics
Although the exact mode of action that causes the irritant effect of FA is not yet well known, since this compound is an aldehyde, it is expected to react easily with the free amino acid groups to produce hydroxymethyl and a free radical (hydrogen proton). It has also been proposed that formaldehyde dehydrogenase becomes saturated when intracellular levels of FA are elevated, limiting natural protection mechanisms and making it easier for this xenobiotic to generate acute or chronic effects in the human body.16,49
Genotoxic effects
Cytotoxicity caused by exposure to FA has been proven through nasal biopsies performed on OEW, in whom chronic inflammation, mild epithelial dysplasia, loss of respiratory cilia, hyperplasia, squamous metaplasia of the epithelium and cancer of the nasopharynx and sinuses were observed.14,50 The toxic effects produced by FA metabolism in the human body that have been identified so far are gene mutation, chromosomal breakage, aneuploidy, epigenetic alterations, oxidative stress, cytotoxicity and induction of cell proliferation.15
In studies using cultures of human cells in vivo, Peteffi et al.26 and Shaham et al.51 showed that FA can produce genotoxicity, as it causes DNA damage and chromosome changes, often expressed as chromosome aberrations, DNA adducts, sister chromatid exchange and micronuclei. Additionally, it has been reported that this compound damages hematopoietic progenitor cells in vitro, which increases its possible relationship with hematological diseases such as acute myeloid leukemia.15,16
Formaldehyde and formic acid as biomarkers of exposure
Although attempts have been made to determine the exogenous concentration of FA in blood, it cannot be used as a biomarker of exposure since only a very low inhaled fraction of FA enters the blood stream. Moreover, the half-life of FA in peripheral blood is 1 to 1.5 minutes, its high reactivity allows it to transform rapidly into other compounds, and the metabolic buffering capacity of the body's nasal cells keeps its levels in blood in the range of 2-3 mg/L.52
Formic acid can be a biomarker of exposure since it is a metabolite of FA excreted in urine; however, its use has been controversial because of its inter-individual variability and the influence of factors such as smoking, diet and nutritional status on the levels of this acid in urine. Also, formic acid can be produced from other substrates of metabolism, so it is not a specific biomarker for detecting exposure to FA. Therefore, Peteffi et al.42 suggested that it could be a biomarker with interferences in its outcome, while Schmid et al.43 concluded that the results of formic acid in urine do not allow assessing exposure to FA, even when the concentration of this pollutant in the working environment is above 50% of the permitted limit.
Genotoxic effects and biomarkers of genotoxicity
Most studies included in the review agree on reporting two biomarkers of genotoxicity: the micronucleus assay on exfoliated cells from the buccal mucosa to visualize local damage, and the comet assay on peripheral blood lymphocytes to identify systemic damage.53 Usually, two genotoxicity tests are done, although the results do not always coincide.
Fenech et al.29 and Chiarella et al.46 do not recommend the use of formic acid in urine or genotoxicity tests since they may be altered by exposure to other xenobiotics and, therefore, they may not be conclusive. However, the studies conducted by these authors were limited to animal models.
Souza & Devi30 and Pira et al.54 agree that the damage caused by FA is directly proportional to years of exposure and concentration in the work environment. This is consistent with Lin et al.,32 who established a relationship between FA concentration and time of exposure, and genotoxic damage.
As for immunotoxicity, Jia et al.,55 Aydin et al.33 and Seow et al.56 reported decreased immune cells and immunoglobulin production, as well as DNA damage. This was evaluated using a comet assay, which suggests that exposure to FA caused immunosuppression in the populations studied, which, in turn, may be associated with diseases of the myeloid system and may explain the mechanism of damage of FA cytotoxicity.
Although genotoxicity biomarkers are not specific for establishing exposure to FA, they provide information on the possible health effect of this pollutant on OEW, taking into account its mechanism of damage, which may facilitate preventive decision-making; therefore, evaluating their usefulness is suggested for individual surveillance. Formic acid in urine as a biomarker does not provide consistent information on exposure to FA, nor does it work to identify cases; on the contrary, it creates confusion and its results can cause the implementation of erroneous intervention and follow-up strategies.
Occupational exposure limits
Although FA is found naturally in the air, there is an increase in its concentration in the most populated urban areas caused mainly by anthropogenic sources. In rural areas, airborne FA concentrations are generally <1 µg/m3, while in urban environments levels are approximately 0.16ppm.14 Garcia-Reynoso et al.57 reported that FA is the environmental pollutant with the highest concentration in Mexico City, increasing the probability of suffering from cancer; consequently, this result is associated with a decrease in life expectancy of the inhabitants of this city.
Regulatory agencies have established permissible limits for FA in work environments according to time of exposure to protect workers' health (Table 3). In 2012, the European Chemicals Agency Risk Assessment Committee concluded that the lowest adverse effects concentration of FA is 2ppm, causing histopathological lesions, polypoid adenomas, and cell proliferation.58
Table 3 Permissible limits for exposure to formaldehyde.
| Agency | Permissible limit | ppm | mg/m3 |
|---|---|---|---|
| American Conference of Governmental Industrial Hygienists | TLV - TWA * | 0.1 | - |
| American Conference of Governmental Industrial Hygienists | TLV - STEL t | 0.3 | - |
| Occupational Safety and Health Administration | PEL - TWA | 0.75 | 0.93 |
| Occupational Safety and Health Administration | STEL | 2 | - |
| National Institute of Occupational Safety and Health | REL - TWA | 0.016 | - |
| National Institute of Occupational Safety and Health | CEILING * | 0.1 | - |
TLV: threshold limit value; TWA: time weighted average; STEL: short term exposure limit; PEL: permissible exposure limit; REL: recommended exposure limit.
* Maximum concentration for 8 hours per day and 40 hours per week.
† Concentration that should not be reached when working for 15-minute periods, maximum 4 times per day, leaving a
1-hour rest period between exposures.
‡ Concentration to which workers should never be exposed during their work shift.
Source: Own elaboration based on the American Conference of Governmental Industrial Hygienists.59
The concentrations reported in most of the articles included in the review exceed the allowable limits proposed by the American Conference of Governmental Industrial Hygienists (TLV-TWA=0.1ppm),59 indicating high exposure to FA in the populations studied.
The selection of the analytical technique to quantify FA depends on the level of sensitivity desired, the technology available, and the possible interferences present in the environment to be evaluated. The most used methods of analysis are NIOSH 350038 and NIOSH 2016,40 which use the ultraviolet-visible spectrophotometry (UV-Vis) analytical technique. The studies by Peteffi et al.26 and Peteffi et al.,27 carried out in 20l6 in Brazil, utilized passive monitoring systems because they are easier to use since they do not require a sampling pump.
Control strategies
The studies included in the review showed the lack of control systems, such as extraction booths and ventilation systems, that reduce the concentration of FA in work environments.41 In people who perform body dissection activities in anatomy laboratories, eye and nasal irritation and the sensation of fatigue seem to be constant symptoms.31 Even though the effect of FA can be reduced by using personal protective equipment, students and professors of the health area rarely use it,60 and the same thing happens among workers in other work environments.30 As described by Saowa-kon et al.12, only a small proportion of the population reported wearing goggles and, despite their use, they did not perceive the decrease in eye irritation; therefore, it is concluded that their use does not minimize the worker's exposure to FA. For their part, workers included in the Costa et al. study8 reported not wearing goggles or respiratory protection, as these elements interfered with activities such as note taking, material handling, and communication.
Even though exposure to FA can be monitored, it is always recommended to consider the substitution of this compound by less hazardous substances for health, considering that it is classified as a carcinogenic substance for humans. For example, Rocha-Ferreira et al.61 studied a solution of ethanol and glycerol for the preservation of corpses, achieving results that can be replicated.
Preference should be given to the establishment of closed processes in which the worker has no contact with this pollutant and perform engineering controls that minimize its presence in the work environment. Furthermore, these measures should be included when technology and production systems are updated, applying control strategies according to their hierarchy: first at the source, then in the environment, and finally in the worker.
Conclusions
According to the Colombian regulations,1,62,63 employers must periodically evaluate the levels of exposure of workers to chemicals considered a priority. In other words, potentially carcinogenic agents should be monitored to determine the risk they pose to OEW, the effectiveness of the control systems in place, and perform relevant medical follow-up. However, care guidelines for occupational exposure to FA have not yet been established.
Strategies implemented as part of the surveillance system often include workplace environmental measurements, spirometry, and formic acid test in urine, although the latter is not a reliable biomarker of FA exposure. Therefore, based on the articles included in this review, we propose a sequence of actions to carry out the epidemiological surveillance of OEW and present biomonitoring alternatives for this population (Figure 3).
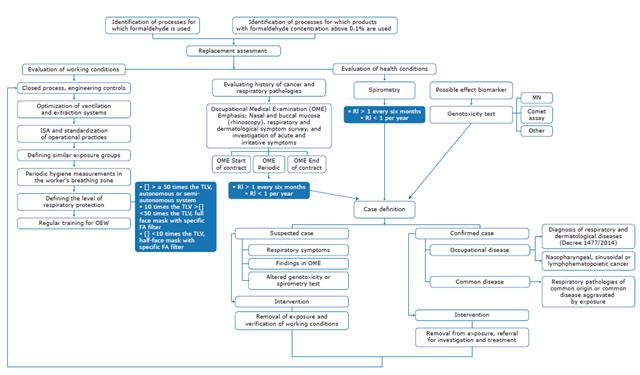
FA: formaldehyde; JSA: job safety analysis; OEW: occupationally exposed workers; OME: occupational medical examination; TLV: threshold limit value; RI: risk index; MN: macronuclei. Source: Own elaboration.
Figure 3 Flowchart for epidemiological surveillance of workers occupationally exposed to formaldehyde.
Additionally, acute symptoms and chronic injuries should be monitored, and the use of respiratory protection should be implemented whenever workers are exposed to FA. This seeks to reduce the damages caused by this pollutant since harmful effects could occur even if exposure is at low concentrations.14,24,26
Occupational environmental measurements should be made in the breathing area of the OEW; no fixed environmental measurements should be made, as the results are statistically different. FA in blood and formic acid in urine are not reliable biomarkers of exposure to this compound, since they are not very sensitive and specific, and their results can be affected by different factors. Genotoxicity markers are a better option for identifying the mechanism of FA damage and for medical follow-up of OEW.
The proper management of the risk derived from occupational exposure to FA, as well as the appropriate medical follow-up of these workers, requires the implementation of a series of interdisciplinary actions that allow the creation of a comprehensive occupational surveillance system of OEW to this substance, taking into account that it is currently the most used preservative to manufacture multiple products.

















