Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.33 no.1 Bogotá Jan./June 2007
Artículo de Revisión
Phylogenetic studies and modern classification of the Pyraloidea (Lepidoptera)
Estudios filogenéticos y clasificación actual de los Pyraloidea (Lepidoptera)
M. Alma Solis
Systematic Entomology Laboratory, USDA. Smithsonian Institution, P.O. Box 37012. National Museum Natural History, E-517, MRC 168. Washington, D.C. 20013-7012. alma.solis@ars.usda.gov
Abstract. Pyraloidea, the third largest superfamily of the Lepidoptera, is comprised of two families - Pyralidae and Crambidae. The history of families previously placed in the Pyraloidea is discussed. The group now includes about 16,000 species worldwide. Morphologically, the superfamily is defined by a basally scaled proboscis and the presence of abdominal tympanal organs. The larvae of many species are economically important pests of crops (e. g.: sugarcane, corn, rice), and stored products such as seeds and grains. Currently 22 subfamilies comprise the Pyraloidea; only the 19 subfamilies that occur in the Western Hemisphere are discussed. There is a paucity of recent research using cladistic methods and phylogenetic analyses across all taxa.
Key words. Pyralidae. Crambidae. Morphology. Cladistic analyses. Searchable on-line catalogs.
Resumen. Pyraloidea, la tercera superfamilia más grande de Lepidoptera, está compuesta por dos familias Pyralidae y Crambidae. Se discute la historia de las familias previamente ubicadas en Pyraloidea. Actualmente el grupo incluye cerca de 16.000 especies en el mundo. Morfológicamente, la superfamilia se define por una proboscis con escamas basales y por la presencia de órganos timpánicos abdominales. Las larvas de muchas especies son plagas de cultivos económicamente importantes (p. ej.: caña de azúcar, maíz, arroz) y de productos almacenados tales como granos y semillas. Actualmente los Pyraloidea comprenden 22 subfamilias, solo se discuten las 19 subfamilias que se encuentran en el hemisferio occidental. Hay pocas investigaciones recientes que usen métodos cladísticos y análisis filogenéticos en todos los taxones.
Palabras clave. Pyralidae. Crambidae. Morfología. Análisis cladístico. Consulta de catálogos en línea.
Pyraloidea, the third largest superfamily of the Lepidoptera following Noctuoidea and Geometroidea, are comprised of two families - Pyralidae and Crambidae. The group includes about 16,000 species worldwide, with greatest richness in the tropics (Fig. 1). Morphologically, the superfamily is defined by a basally scaled proboscis and the presence of abdominal tympanal organs. The larvae of many species are economically important pests of crops (e. g.: sugarcane, corn, rice), and stored products such as seeds and grains (Solis 1997).
The familial composition of the Pyraloidea (Table 1) has changed markedly over the past 30 years, with a general decrease in the number of families. Pterophoridae, Alucitidae, Thyrididae, Hyblaeidae, Oxychirotidae and Tineodidae formerly included in the Pyraloidea, are now in their own superfamilies with unresolved affinities (Fletcher and Nye 1984; Minet 1986; Nielsen 1989; Common 1990). Dudgeoneidae is now assigned to Cossidae, and Lathrotelidae, proposed by Clarke (1971) for a monotypic genus from the Pacific islands, is now considered to belong to the Acentropinae in the Crambidae (Minet 1991).
Pyraloidea moths are ditrysian moths characterized by the following morphological features: paired tympanal chambers on sternite 2, each with a tympanum and a conjunctiva (Fig. 2), a maxillary palpus that is usually present, a basally scaled proboscis, veins R3 and R4 of the forewing stalked or fused, and Sc+R1 and Rs of the hindwing anastomosed distad of the discal cell.


Although published catalogs on subfamilies are listed below, there are two searchable on-line catalogs of the Pyraloidea. The LepIndex, at The Natural History Museum, London, United Kingdom, is based on their card catalog (Beccaloni et al. 2003). The other, GLOBIZ, at the State Museum of Zoology, Dresden, Germany, is compiled and updated by current researchers in the Pyraloidea (Nuss 2006).
Pyraloidea currently is divided into two families based primarily on two distinct tympanal organ types of the adult abdomen, but characters of the larvae also confirm this division. The Pyralidae have a tympanal case that is almost closed, the conjunctiva and tympanum are in the same plane, and the praecinctorium is absent. The Crambidae have a tympanal case that is open with a wide anteromedial aperture, the conjunctiva and tympanum are in a different plane and meet at a distinct angle, and the praecinctorium is present (Minet 1981; Maes 1995). Börner (1925) was the first to recognize the difference between the two groups in the Pyraloidea, and Munroe (1972, 1973, 1976) proposed the informal groups, Pyraliformes and Crambiformes based on the major differences between the two types of tympanal organs. Minet (1983) subsequently elevated Munroe's groups to the Pyralidae and Crambidae based on an extensive study of tympanal organs in Lepidoptera.
Although some contemporary lepidopterists do not recognize the two families as distinct because they “cannot be separated by external diagnostic characters” (Landry 1995), characters of the tympanal organs and larvae are certainly external. From a cladistic perspective, most of the structures of the crambid type of tympanal organ represent synapomorphies. the most convincing synapomorphy for Pyralidae is the sclerotized ring around the base of seta SD1 on segment A8 of the larva (Fig. 3). In a few genera in both Crambidae and Pyralidae, tympanal organs are absent or reduced (e. g.: Lathroteles (Minet 1991); Michaelshaffera (Solis 1998)), and a few pyralid species lack the sclerotized ring on A8, although a shiny white translucent ring is present if it is not sclerotized (Etiella zinckenella (Solis 1999a); laboratory reared Galleria mellonella, personal observation). A molecular study testing the utility of the period gene on three species of Pyraloidea showed two crambid species as sister group to one pyralid species (Regier et al. 1998)

The larvae of Pyraloidea exhibit diverse behavioral characteristics. Some are concealed feeders that fold, roll, web, or tie leaves together to form a shelter. Others are borers of monocots and can be found in the stems, roots, shoots, buds, fruits, or galls. A few mine leaves, make cases, or live in the nests of ants, bees, and wasps (selected references in Munroe and Solis 1999; Solis 1997). Some groups of pyraloids have adapted to aquatic environments (Lange 1956; Munroe 1972; Solis in press) where they mostly are plant feeders, but some have been observed as predators on other insects. The larvae of pyraloid species are scavengers, feeding on stored products or the fecal matter of sloths (Waage and Montgomery 1976) and bats (Solis and Mitter 1992). Pyraloids feed on a wide diversity of plants (Solis 1999a; Robinson et al. 2002), including monocots, dicots, and algae (Solis in press); one subfamily specializes on ferns (Solis et al. 2004b, 2005 et al.; Yen et al. 2004). Pyraloid larvae can be distinguished from other Lepidoptera by the following combination of characters: two prespiracular setae on the prothorax, crochets in a complete circle or penellipse (with the exception of aquatic immatures where they are usually in two rows), and three subventral setae on abdominal segments 3 to 6. Noctuids and carposinids also have two prespiracular setae on the prothorax, but noctuids have crochets in a mesoseries, and carposinids have four or more subventral setae on abdominal segments three to six (Solis 1999a). The literature on the morphology and biology of immatures is scattered, but see Allyson (1981, 1984) for Nearctic pyraloid larvae. There are very few comprehensive studies on the pupae (Mosher 1916; Patocka and Turcani 2005).
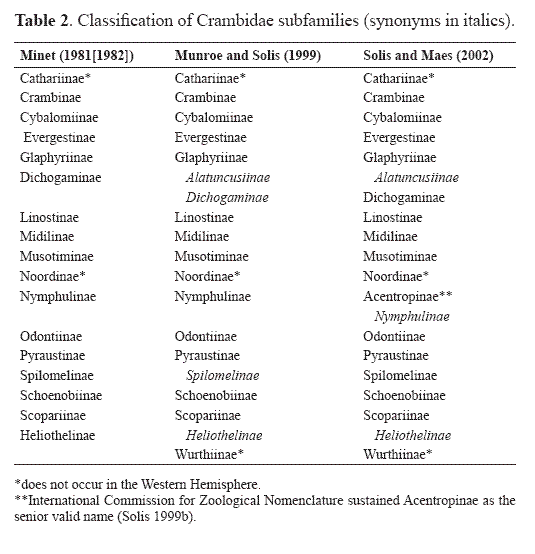
Family Crambidae
Currently 17 subfamilies are recognized in the Crambidae (Table 2). Three subfamilies, Cathariinae, Noordinae, and Wurthiinae do not occur in the Western Hemisphere and are not discussed here (see Munroe and Solis 1999). Solis and Maes (2002) proposed a preliminary hypothesis of relationships based on adult characters only (Fig. 4). The Spilomelinae and Pyraustinae, previously hypothesized to be closely related, are not. Evidence suggests that further phylogenetic work on polyphyletic groups (e. g.: Spilomelinae) will reveal more subfamilial taxa, and some of the subfamilies will be shown to be paraphyletic in relation to other groups. For example, the subfamilies Pyraustinae + Evergestinae + Odontiinae + Glaphyriinae are likely paraphyletic to each other (Solis and Maes 2002). The Crambidae is the larger family with just under 10,000 described species worldwide. In addition to the tympanal characters mentioned previously [a tympanal case that opens anteromedially, a conjunctiva and tympanum that are not in the same plane, and the presence of the praecinctorium] one larval character defines the family: 1 or 2 L setae on A9 (Hasenfuss 1960; Minet 1981, 1983, 1985). (table 3)

Subfamily Spilomelinae. Spilomelinae is the largest subfamily in the Pyraloidea, and until the 1980s it was subsumed within Pyraustinae. Spilomelinae was resurrected by Minet (1981) based on several morphological characters. However, as presently defined, it is polyphyletic, and upon further study it will be separated into monophyletic sister groups. Very few studies using cladistic methods have been conducted (i. e.: black and white Diaphania (Clavijo 1990), New World Omiodes (Gentili and Solis 1998), and Australian Glyphodes and related genera (Sutrisno 2002a, b)). Sutrisno et al. (2006) conducted a molecular study using two mitochondrial genes (COI and COI ) and the nuclear gene EF-1α and found that Glyphodes was monophyletic, although previous morphological results were not congruent with this result. Spilomelinae is comprised of hundreds of genera, including Neoleucinodes and Leucinodes that are major pests of solanaceous crops in the New World and Old World respectively; Lineodes, exhibits great species diversity in Peru and species feed on potatoes; Udea, that includes the greenhouse leaf tier; Desmia, that includes leaf rollers on grapes; Herpetogramma, Hymenia, and Spoladea, that are polyphagous on a wide variety of crops; Diaphania, a very diverse, polyphyletic group with major pests of cucurbits in the Neotropics; Omiodes and Maruca, that include major pest of legumes worldwide; Palpita and Conogethes, that include pests of peaches and conifers in the Old World, Azochis species are shoot borers in coffee and other tropical trees; Siga, a group with bright green wings with “windows” that exceed 100 mm in wingspan; Agathodes and Terastia, that are borers and webbers of Erythrina shoots and flowers; and the mimetic Hyalea and Trichaea, with dioptid and hymenopterous models respectively (Munroe and Solis 1999).
Subfamily Crambinae. The Crambinae is the second largest subfamily in Crambidae. Although many studies have been conducted at lower taxonomic levels owing to the economic importance of various included groups (Hampson 1895; Box 1931; Bleszynski 1969, 1970; Gaskin 1975, 1985; Schouten 1992; Landry 1995), the relationship of Crambinae to other subfamilies within the Crambidae remains unresolved (Solis and Maes 2002). This subfamily includes stem-boring genera such as Myelobia whose larvae attack bamboo, and Chilo, Diatraea, and Eoreuma that attack sugarcane (Bleszynski 1969; Solis 2004); Chilo and Diatraea also attack rice. Rootand leaf-feeding is characteristic of Crambus, a genus that includes many sod webworms that emerge at night to feed on the grasses, and live in silk-lined tunnels in the soil during the day. A cladistic study of Nearctic species was conducted by Landry (1995).
Subfamily Pyraustinae. The Pyraustinae is the third largest subfamily in the Crambidae and has been the focus of various studies by Maes (e. g.: 1987, 1994, 1995, 2002) primarily on the fauna of Africa and the Palearctic region. It is well defined by characters of the tympanal organs (atrophied spinula and venulae and a narrow fornix tympani) and the male genitalia (valvae with sellae (a medially direct clasper with modified setae known as edita), the male mesothoracic tibiae (with a longitudinal groove that holds androconial scales), and the collar or antrum of the female genitalia (often with spines). This subfamily includes Loxostege, that may migrate in swarms and whose larvae may defoliate fields; Achyra, that includes major polyphagous pests of crops (Maes 1987); and Ostrinia nubilalis (Hübner, 1796) (Mutuura and Munroe 1970), that was introduced into the U.S. and has become a major pest on corn and other crops (Munroe and Solis 1999). There has been no higher level phylogenetic work on genera.
Subfamily Acentropinae. Acentropinae, formerly called Nymphulinae (Solis 1999b), has many species that are truly aquatic as immatures either with gills protruding from the integument or using a plastron for oxygen exchange. The larvae are mostly herbivorous, but a few have been shown to be predators of Simuliidae in Brazil (Yen 2004b; Solis in press). Most of the species in Petrophila are scrapers, feeding on algae and diatoms on the surfaces of rocks or other submerged objects, but other species feed on submerged plants, such as P. drumalis (Dyar, 1906), that feeds on the rootlets of water lettuce (Pistia) (Habeck and Solis 1994). Yoshiyasu (1985) defined the Acentropinae based on the protruding spiracles of the pupae on the second to fourth segments, but these characters have since been found to occur in members of the Musotiminae (Yen et al. 2004). Minet (1985) defined the subfamily by the presence of swollen scoloparia in the tympanal organs. Munroe (1972) reviewed the Nearctic Acentropinae, but his higherlevel nomenclature is now outdated. There are no confirmed synapomorphies for this subfamily. There are several catalogs from China (Speidel and Mey 1999; You et al. 2002) and Chen has conducted several generic revisions from China (e. g.: 2006). Shen-Horn Yen (personal communication) is conducting a worldwide study of the genera of the Acentropinae.
Subfamilies Odontiinae, Evergestinae, Schoenobiinae, Scopariinae, Cybalomiinae, Musotiminae.Other smaller subfamilies of the Crambidae, such as Odontiinae, Evergestinae, Schoenobiinae, Scopariinae, Cybalomiinae, and Musotiminae, occur worldwide but are not very speciose in the Neotropics. Odontiinae was defined by Leraut and Luquet (1982) by the scalelike sclerotizations at the base of the vinculum of the male genitalia and two sets of non-deciduous scales on sternite VII . The Evergestinae is even less well known; Minet (1981) defined the subfamily by derived characters of the tympanal organs. Schoenobiinae usually have long, narrow brown wings, but Rupela, a pest of rice, have brilliant white wings. Schoenobiinae are defined by a membranous area on sternite VII covered by stiff scales from the posterior margin of sternite VI (Common 1990); the presence of platelike, scale-bearing coremata flanking the vinculum (Lewvanich 1981); a process on the lateral margin of the gnathos (Maes 1998); and a highly bilobed praecinctorium with a pair of circular formations called tubercula by Minet (1981, 1985). Larvae bore into grasses found in marshes and Scirpophaga in the Old World, and Rupela are pests of rice in the New World. Scopariinae moths are usually very small with black and white scales (Munroe 1972; Nuss 1999). Minet (1981) defined the subfamily by its longitudinal praecinctorium, reduced saccus tympani, and a well-developed gnathos in the male genitalia. Heliothelinae, currently is considered a synonym of Scopariinae, but this has been questioned by Nuss (1998) and Nuss et al. (1998). The most speciose genera, Eudonia and Scoparia, reach their greatest diversity on oceanic islands (Nuss 1999). The larvae tunnel in roots and stems of mosses and lycopods or feed on roots of vascular plants (Munroe and Solis 1999). Cybalomiinae is not well defined; nine species have been described in the Neotropics, but all but one belong in other subfamilies. Their placement in this subfamily is not tenable and is under investigation by Solis. Some larvae are known to feed on Cruciferae (Luquet and Minet 1982). Musotiminae is a subfamily that was extracted from Acentropinae by Munroe (1972) based on the fact that most known species feed on ferns and do not have aquatic immatures. They have not been studied on worldwide basis, but Solis et al. (2004b) and Yen et al. (2004) have been transferring taxa to the Musotiminae and describing new taxa that feed on the Old World Climbing fern in southeast Asia and other ferns in the New World. Musotimines are defined by antennae that are laterally compressed, R2 stalked with R3+4, aedeagus with a reduced coecum, and tympanal cases enlarged (Minet 1981; Yoshiyasu 1985; Yen et al. 2004), but the relationship of Musotiminae with other subfamilies in Crambidae is unknown. Phillips and Solis (1996) studied Neurophyseta in Costa Rica; and Solis et al. (2005) described a new genus from Costa Rica that are leaf miners on a fern.
Subfamilies Linostinae, Midilinae, Glaphyriinae. Some subfamilies occur primarily in the Neotropics: Linostinae, Midlinae, and Glaphyriinae. The Linostinae is comprised of three species of Linosta. Although the characters that define this group are mainly absence of characters or secondary losses (reduced labial palpi and lack of maxillary palpi, proboscis, ocelli and chaetosemata (Munroe and Solis 1999)), they are easily recognizable by white forewings with distinct black lines. The immature stages are unknown. The Midilinae are spectacular moths with hyaline discal spots and sinuate terminal margins on red, green or yellow forewings; there are 47 described species (Munroe 1970). Minet (1981, 1985) and Munroe and Solis (1999) defined the subfamily on the basis of the reduced tympanal organs, a short praecinctorium, chaetosemata on the head often well delineated from the surrounding area, and an ostium bursae lacking a signum but with diffuse patches of spinules. The larvae are known to bore into stems and roots of Araceae. Solis and Adamski (1998), in their study of the Costa Rican Glaphyriinae, defined the subfamily by the absence of chaetosemata on the head, gnathos of the male genitalia, and the presence of a sclerotized antrum in the female genitalia. That study revealed that a generic revision of all 200 species in the Western Hemisphere is greatly needed. A few species of Hellula are pests of crucifers and occur worldwide. There has been no higher-level phylogenetic analysis of this subfamily.
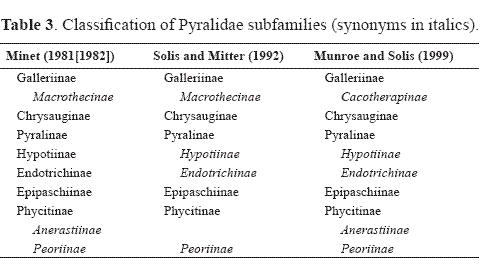
Family Pyralidae
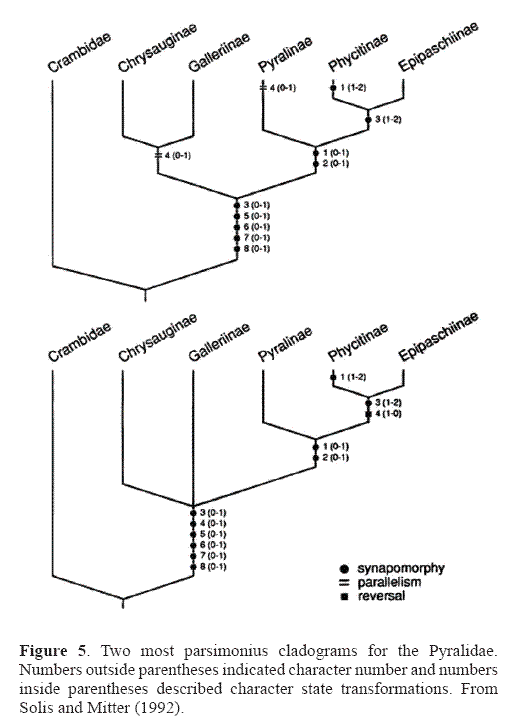
The Pyralidae is the smaller of the two families with a little less than 5,000 described species. It includes five subfamilies: Phycitinae, Epipaschiinae, Pyralinae, Chrysauginae, and Galleriinae. The monophyly of the Pyralidae is supported by the closed bullae tympani (Minet 1981, 1985), forewing veins R3 and R4 completely fused or stalked at their base, uncus arms in the male genitalia (Solis and Mitter 1992), and larvae with a sclerotized ring at the base of SD1 on A8 (Hasenfuss 1960). A phylogeny of the pyralid subfamilies proposed by Solis and Mitter (1992) (Fig. 5) shows support for the sister group relationship of the Epipaschiinae and Phycitinae, but the galleriines and chrysaugines were found to be unresolved basally.
Subfamily Galleriinae. Galleriinae occur worldwide and are defined by a lack of a gnathos (Whalley 1964; Munroe 1972). Although two genera of Chrysauginae also lack a gnathos (Cashatt 1968) it presumably has been lost independently in Galleriinae and Chrysauginae. Recently research on the musculature (Solis unpublished) indicates that a remnant of a gnathos may be present but extremely difficult to see. Galleriine pupae have a prominent median ridge on the dorsum of the thorax and abdomen (Mosher 1916), and the larvae of most species have a sclerotized ring around SD1 of A1 (Hasenfuss 1960), except for Omphalocera and Thyridopyralis (Solis 1989; Solis and Mitter 1992). A world catalog of this subfamily was compiled by Whalley (1964), but no phylogenetic analyses have been conducted. This subfamily includes the bee or wax moth, Galleria mellonella (L., 1758), that feeds on honeycombs, and the rice moth, Corcyra cephalonica (Stainton, 1866). Other genera, such as Aphomia, have symbiotic relationships with other hymenopterans, and Paralipsa species are stored product pests worldwide (Munroe and Solis 1999).
Subfamily Chrysauginae. Chrysauginae are primarily neotropical (Cashett 1968). The monophyly of the subfamily is supportd by a a sclerotized ring at the base of SD1 on the metathorax (Hasenfuss 1960), but this feature is absent in Azamora (Solis 1989). A checklist of the Chrysauginae in the Western Hemsiphere was compiled by Solis et al. (1995), but no phylogenetic analyses have been conducted. Four Australian genera were placed tentatively in this subfamily by Shaffer et al. (1996), but study of the adults did not confirm this, and the larvae are unknown. In the Western Hemisphere, this subfamily is home to the sloth moths. Adults of these species live in the fur, and the larvae feed on the sloth's dung (Waage and Montgomery 1976). Sloth moths are placed in several genera (Cryptoses, Bradypodicola, Bradypophila) that are paraphyletic with respect to each other (being worked on Roe, pers. comm.).
Subfamily Pyralinae. Pyralinae is a worldwide group, but not very speciose in the New World. The subfamily is defined by a corpus bursae in the female genitalia that barely extends cephalad beyond abdominal segment 7 (Solis and Mitter 1992). A checklist of the Western Hemisphere and a morphological review of the Pyralinae was presented by Solis and Shaffer (1995, 1999), but no phylogenetic analyses have been conducted. The subfamily includes the meal moth, Pyralis farinalis L., 1758, and other species that are pests of stored product, leaf feeders, and one species, P. manihotalis Guenée, 1854, that has been reared from bat guano (Solis and Mitter 1992).
Subfamily Epipaschiinae. Epipaschiinae were examined by Solis and Mitter (1992) and Solis (1993), and three synapomorphies were discovered to define it. The third segment of the labial palpus is upturned and pointed, the phallobase of the aedeagus extends beyond the ductus ejaculatorius and is curved ventrally, and there is weakness in the sclerotization at the base of the tegumen that results in the appearance of a separate sclerite. Larvae are known to be leaf rollers, leaf tiers, and leafminers, but their morphology is not well known. The group includes minor pests of avocado (e. g.: Accinctapubes), mahoganies (e. g.: Macalla), and corn (e. g.: Phidotricha). Solis (1993) provided a phylogenetic analysis of 20 genera of the Pococera complex, which consists of 300 species in the Western Hemisphere, representing the first cladistic analysis of a pyraloid group. A similar study of about 500 Old World species is needed. Checklists of Old World and New World epipaschiines have been published (Solis 1992; Solis 1995).
Subfamily Phycitinae. The Phycitinae is the largest subfamily, consisting mostly of small moths worldwide. It is defined by larvae with a sclerotized circle at the base of seta SD1 on the mesothorax (Hasenfuss 1960). The female frenulum has one bristle as in the male (females have two bristles in pyralines and epipaschiines, three in galleriines and chrysaugines), and the ductus seminalis originates in the corpus bursae (in other pyralid subfamilies it originates in the ductus bursae) (Solis and Mitter 1992). Shaffer (1968, 1976) has revised many groups of the Peoriini. The Phycitinae includes many stored product pests, including Plodia, Cadra, Ectomyelois, and Etiella (Solis 1999a). The larvae are leaf rollers and borers in various parts of plants, although a few are known to be predators of Homoptera (e. g.: Laetilia). Cactoblastis cactorum (Berg, 1885), the cactus moth is the classical biological control agent of Opuntia in various countries, but in recent years has become an introduced invasive in the United States (Solis et al. 2004a). The New World Phycitinae were revised by Heinrich (1956), the Nearctic members by Neunzig (e. g.: 1986, 1990, 1997, 2003), and the Old World Homeosoma species by Roesler (1965). Whalley (1970) produced a world catalog of the Phycitinae. Recently, Neunzig and Solis (e.g. 2002, 2004, 2005a,b, 2006) have published a number of papers regarding Costa Rican phycitines, Horak (e. g.: 1998) has published on Australian Phycitinae, and Li and coauthors have published on Chinese genera (e. g.: Li and Ren 2006). Simonsen (pers. comm.) is conducting research on the cactus feeding phycitines. Recent studies on Dioryctria, which are pests of conifers in the Holarctic region, indicates that combining morphological and molecular characters increased nodal support in hypothesize phylogenetic trees (Du et al. 2005; Roe et al. 2006).
Literature Cited
ALLYSO N, S. 1981. Last instar larve of Pyraustini of America north of Mexico (Lepidoptera: Pyralidae). Canadian Entomologist 113: 463-518. [ Links ]
ALLYSO N, S. 1984. Description of last-instar larvae of 22 species of North American Spilomelini (Lepidoptera: Pyralidae: Pyraustinae) with a key to species. Canadian Entomologist 116: 1301-1334. [ Links ]
BECCALONI, G. W.; SCOBLE, M. J.; ROBINSO N, G. S.; PITKIN, B. (eds.). 2003. The Global Lepidoptera Names Index (LepIndex). World Wide Web electronic publication.www.nhm.ac.uk/entomology/lepindex Last revision: 1 February 2005. Last access: 15 May 2007. [ Links ]
BLESZYNSKI, S. 1969. The taxonomy of the crambine moth borers of sugar cane, pp. 11-59. In: Williams, J. R.; Metcalf, J. R.; Mungomery, R. W.; Mather, R. (eds.). Pests of Sugar Cane. New York: Elsevier Publishing Co. [ Links ]
BLESZYNSKI, S. 1970. A revision of the world species of the genus Chilo Zincken. (Lepidoptera: Pyralidae). Bulletin of the British Museum (Natural History) Entomology 25 (4): 101-195. [ Links ]
BÖRNER, C. 1925. Lepidoptera, Schmetterlinge. pp. 382- 421. In: Bröhmer, P. (ed.). Fauna von Deutschland: Berlin: Leipzig, Quelle & Meyer: 561 p. [ Links ]
BOX, H. E. 1931. The crambine genera Diatraea and Xanthopherne (Lep. Pyralidae). Bulletin Entomological Research 22: 1-50. [ Links ]
CAS HATT, E. D. 1968. Revision of the Chrysauginae of North America. Dissertation, Catholic University of America, Washington, D.C. 179 p. [ Links ]
CHEN, F.; SO NG, S.; WU, C. 2006. A review of the genus Eristena Warren in China (Lepidopera: Crambidae: Acentropinae). Aquatic Insects 28 (3): 229-241. [ Links ]
CLARKE, J. F. G. 1971. The Lepidoptera of Rapa Island. Smithsonian Contributions Zoology 56: 1-282. [ Links ]
CLAVIJO A., J. A. 1990. Systematics of black and white species of the genus Diaphania Hübner (1818) (Lepidoptera: Pyralidae: Pyraustinae). Dissertation: Montreal, Canada: McGill University. 215 p. [ Links ]
COMO N, I. F. B. 1990. Pyraloidea, pp. 343-358. In: Moths of Australia. Carlton, Victoria: Melbourne University Press. [ Links ]
DU, Y.; A. D. ROE; F.A.H. SPERLING. 2005. Phylogenetic framework for Dioryctia (Lepidoptera: Pyralidae: Phycitinae) based on combined analysis of mitochondrial DNA and morphology. Canadian Entomologist 137: 685-711. [ Links ]
FLETCHER, D. S.; I. W. B. NYE. 1984. The Generic Names of the Moths of the World. London: Trustees of the British Museum (Natural History): Vol. 5, 185 p. [ Links ]
GAS KIN, D. E. 1975. Revision of the New Zealand Crambini (Lepidoptera: Pyralidae: Crambinae). New Zealand Journal Zoology 2 (3): 265-363. [ Links ]
GAS KIN, D. E. 1985. Morphology and reclassification of the Australasian, Melanesian and Polynesian Glauchocharis Meyrick (Lepidoptera: Crambinae: Diptychophorini). Australian Journal Zoology Supplement Series 115: 1-75. [ Links ]
GENTILI, P.; SO LIS , M. A. 1998. Checklist and key of New World species of Omiodes Guenée with descriptions of four new Costa Rican species (Lepidoptera: Crambidae). Entomologica Scandinavica 28: 471-492. [ Links ]
HABECK, D.; SO LIS M. A. 1994. Transfer of Petrophila drumalis (Dyar) to Argyractis based on immature and adult characters with a larval description of Argyractis subornata (Hampson) (Lepidoptera: Crambidae: Nymphulinae). Proceedings Entomological Society Washington 96 (4): 726- 734. [ Links ]
HAM PSO N, G. F. 1895. On the classification of the Schoenobiinae and Crambinae, two subfamilies of moths, of the family Pyralidae. Proceedings of the Zoological Society of London 1895: 897-974. [ Links ]
HAS ENFUSS , I. 1960. Die Larvalsystematik der Zünsler. Berlin: Academie Verlag: 263 p. [ Links ]
HEINRICH, C. 1956. American moths of the subfamily Phycitinae. Bulletin of the United States National Museum 207: 1-581. [ Links ]
HORAK, M. 1998. A reassessment and review of the Australian genus Cternomeristis Meyrick (Lepidoptera: Pyralidae: Phyctinae). Entomologica Scandinavica 28: 445-470. [ Links ]
LANDRY, B. 1995. A phylogenetic analysis of the major lineages of the Crambinae and of the genera of the Crambini of North America (Lepidoptera: Pyralidae). Memoirs of the American Entomological Institute 1:1-242. [ Links ]
LANGE, H. 1956. Aquatic Lepidoptera, pp. 271-288. In: Usinger, R. L. (ed.). Aquatic Insects of California. University of California Press. Berkeley. 508 p. [ Links ]
LERAUT, P.; LUQUET, G. C. 1982. Statut de quelques genres et espèces d'Odontiinae paléarctiques et description de quatre nouveaux taxa (Lep. Crambidae). Linneana Belgica 8 (12): 527-555. [ Links ]
LEWVANICH, A. 1981. A revision of the Old World species of Scirpophaga (Lepidoptera: Pyralidae). Bulletin of the British Museum (Natural History) Entomology 42 (4): 185-298. [ Links ]
LI, H.-H.; REN, Y.-D. 2006. The genus Thiallela Walker, 1863 in China (Lepidoptera: Pyralidae: Phycitinae). Entomological News 117 (3): 323-331. [ Links ]
LUQUET, G. C.; MI NET, J. 1982. Decouverte dans le Vaucluse d'une nouvelle espèce de Pyrale et designation de l'espècetype du genre Krombia Chrétien (Lepidoptera, Crambidae, Cybalomiinae). Alexanor 12 (7): 317-326. [ Links ]
MAES, K. V. N. 1987. Revisionary notes on the genus Achyra Guenée with a new synonym and the description of Achyra takowensis sp. n. (Lepidoptera: Pyralidae, Pyraustinae) (Studies on Pyralidae I). Nota Lepidopterologica 10 (3): 175- 182. [ Links ]
MAES, K. V. N. 1994. Some notes on the taxonomic status of the Pyraustinae (sensu Minet 1981 [1982]) and a check list of the Palearctic Pyraustinae (Lepidoptera, Pyraloidea, Crambidae). Annales de la Société Royale Entomologique de Belgique 130: 159-168. [ Links ]
MAES, K. V. N. 1995. A comparative morphological study of the adult Crambidae (Lepidoptera, Pyraloidea). Annales de la Société Royale Entomologique de Belgique 131: 383-434. [ Links ]
MAES, K. V. N. 1998. On the morphology of the gnathos in the Pyraloidea (Lepidoptera). Entomological Scandinavica 28 (4): 381 390. [ Links ]
MAES, K. V. N. 2002. On the systematic position of some Paleaearctic Pyraustinae (Pyraloidea: Crambidae). Nota Lepidopterologica 24 (4): 51-57. [ Links ]
MINET, J. 1981 (1982). Les Pyraloidea et leurs principales divisions systématiques. Bulletin de la Société Entomologique de France 86: 262-280. [ Links ]
MINET, J. 1983. Étude morphologique et phylogénétique des organes tympaniques des Pyraloidea. 1. Généralitès et homologies (Lepidoptera Glossata). Annales de la Société Entomologique de France 19 (2): 175-207. [ Links ]
MINET, J. 1985. Étude morphologique et phylogénétique des organes tympaniques des Pyraloidea. 2 - Pyralidae, Crambidae, première partie (Lepidoptera Glossata). Annales de la Société Entomologique de France 21 (1): 69-86. [ Links ]
MINET, J. 1986. Ébauche d'une classification moderne de l'ordre des Lépidoptères. Alexanor 14 (7): 291-313. [ Links ]
MINET, J. 1991. Tentative reconstruction of the ditrysian phylogeny (Lepidoptera: Glossata). Entomological Scandinavica 22: 69-95. [ Links ]
MOSHER, E. 1916. A classification of the Lepidoptera based on characters of the pupa. Bulletin of the Illinois State Laboratory of Natural History 12: 15-159. [ Links ]
MUNROE, E. 1970. Revision of the subfamily Midilinae (Lepidoptera: Pyralidae). Memoirs of the Entomological Society of Canada 74: 1-94. [ Links ]
MUNROE, E. 1972. (Fasc. 13.1A-1B), 1973 (Fasc. 13.1C), 1976 (Fasc. 13.2A-B). Pyraloidea. Pyralidae (in part). In: Dominick, R. B.; Ferguson, D. C.; Franclemont, J. G.; Hodges, R. W.; Munroe, E. G. (eds.). The Moths of America North of Mexico. London: E.W. Classey, Ltd. and The Wedge Entomological Research Foundation: 1- 304, 1-150. [ Links ]
MUNROE, E.; SO LIS , M. A. 1999. Pyraloidea, pp. 233-256. In: Kristensen, N. (ed.). Lepidoptera, Moths and Butterflies, Vol. 1, Arthropoda, Insect, Vol.4, Part 35. Handbook of Zoology. Walter de Gruyter & Co. Berlin. 491 p. [ Links ]
MUTUURA, A.; MUNROE, E. M. 1970. Taxonomy and distribution of the European corn borer and allied species: genus Ostrinia Hübner (Lepidoptera: Pyralidae). Memoirs of the Entomological Society of Canada 71: 1-112. [ Links ]
NEUNZIG, H. H. 1986. (Fasc. 15.2), 1990 (Fasc. 15.3), 1997 (Fasc. 15.4), 2003 (Fasc. 15.5). Pyraloidea, Pyralidae (in part). In: Dominick, R. B.; Ferguson, D. C.; Franclemont, J. G.; Hodges, R. W.; Munroe, E. G. (eds.). The Moths of America North of Mexico. Lawrence, Kansas: Allen Press, Inc.: 1-112, 1-165, 1-157, 1-338. [ Links ]
NEUNZIG, H. H. 1987. Pyralidae (Pyraloidea), pp. 462-494. In: Stehr, F. W. Immature Insects. Dubuque, Iowa: Kendall Hunt Publishing Co. [ Links ]
NEUNZIG, H. H.; SO LIS , M. A. 2002. The Ceracanthia complex (Lepidoptera: Pyralidae: Phycitinae) in Costa Rica. I. Ceracanthia. Proceedings Entomological Society of Washington 104 (4): 837-855. [ Links ]
NEUNZIG, H. H.; SO LIS , M. A. 2004. Exguiana, a new genus of Neotropical phycitines (Lepidoptera: Pyralidae). Proceedings of the Entomological Society of Washington 106 (3): 544-563. [ Links ]
NEUNZIG, H. H.; SO LIS , M. A. 2005a. Tumoriala, a new neotropical phycitine genus (Lepidoptera: Pyralidae). Proceedings of the Entomological Society of Washington 107 (1): 84-89. [ Links ]
NEUNZIG, H. H.; SO LIS , M. A. 2005b. A review of the genus Difundella (Lepidoptera: Pyralidae: Phycitinae). Proceedings of the Entomological Society of Washington 107 (2): 303- 314. [ Links ]
NEUNZIG, H. H.; SO LIS , M. A. 2006. Four new species of Costa Rican Phycitinae (Lepidoptera: Pyralidae). Proceedings of the Entomological Society of Washington 108 (4): 953- 963. [ Links ]
NIELSEN, E. S. 1989. Phylogeny of major lepidopteran groups. In: Fernholm, B.; Bremer, K.; Jörnvall, H. (eds.). The Hierarchy of Life. Copenhagen: Elsevier Science Publishers: 281-294. [ Links ]
NUSS , M. 1998. The Scopariinae and Heliothelinae stat. Rev. (Lepidoptera: Pyraloidea: Crambidae) of the Oriental Region: a revisoinal synopsis with descriptions of new species from the Phillippines and Sumatra. Nachrichten des entomologischen Vereins Apollo Supplement 17: 475-528. [ Links ]
NUSS , M. 1999. Revision der Gattungen der Scopariinae, Lepidoptera: Pyraloidea, Crambidae. Nova supplementa Entomologica 13: 1-151. [ Links ]
NUSS , M. 2006. GLOBIZ, State Museum of Zoology, Dresden, Germany. http://globiz.sachsen.de/pyrdat_prototype. Last revision: 16 May 2006. Last access: 1 May 2007. [ Links ]
NUSS , M.; KARSHOLT, O; MEYER, M. 1998. A taxonomic revision of the Scopariinae from the Macaronesian Region (Lepidoptera: Pyraloidea: Crambidae). Entomological Scandinavica 28: 509-551. [ Links ]
PATOCKA, J.; TURCANI, M. 2005. Lepidoptera Pupae, Central European Species. Apollo Books. Denmark. volumes 542p., Plates 321 p. [ Links ]
PHILLIPS, E.; SO LIS , M. A. 1996. Neurophyseta (Lepidoptera: Crambidae) de Costa Rica. Revista de Biología Tropical 44 (2): 693-717. [ Links ]
REGIER, J. C.; FANG, Q. Q.; MI TTER, C.; PIEGLER, R.S.; FREIDLANDER, T. P.; SO LIS , M. A. 1998. Evolution and Phylogenetic Utility of the period gene in Lepidoptera. Molecular Biology and Evolution 15 (9): 1172-1182. [ Links ]
ROBINSO N, G. S.; ACKERY, P.R.; KITCHING, I. J.;BECCALONI, G. W.; HERNANDEZ, L. M. 2002. Hostplants of the moth and butterfly caterpillars of America North of Mexico. Memoirs of the American Entomological Institute 69: 1-824. www.nhm.ac.uk/entomology/hostplants Last revision: 25 March 2002. Last access: 25 April 2007. [ Links ]
ROESLER, R. U. 1965. Untersuchungen über die Systematik und Chorologie des Homoeosoma-Ephestia-K[o]mplexes (Lepidoptera: Pyralidae). Saarbrücken: Dissertation Saarbrücken (1964) 265 p. [ Links ]
ROE, A. D.; STEIN, J.D.; GILLETTE, N. E.; SPERLING, F.A.H. 2006. Identification of Dioryctria (Lepidoptera: Pyralidae) in a Seed Orchard at Chico, California. Annals of the Entomological Society of America 99 (3): 433-448. [ Links ]
SCHOUTEN, R. T. A. 1992. Revision of the genera Euchromius Guenée and Miyakea Marumo (Lepidoptera: Crambidae: Crambinae). Tijdschrift voor Entomologie 135: 191-274. [ Links ]
SHAFFER, J. C. 1968. A revision of the Peoriinae and Anerastiinae (auctorum) of America north of Mexico (Lepidoptera:Pyralidae). Bulletin of the United States National Museum 280: 1-124. [ Links ]
SHAFFER, J. C. 1976. A revision of the neotropical Peoriinae (Lepidoptera: Pyralidae). Systematic Entomology 1: 281-331. [ Links ]
SHAFFER, M.; NIELSEN, E. S.; M. HORAK. 1996. Pyraloidea, pp. 164-199 In: Nielsen, E.S.; Edwards, E. D.; Rangsi, T. V. (eds.). Checklist of the Lepidoptera of Australia. Monographs on Australian Lepidoptera 4: 1-529. [ Links ]
SOLIS , M. A. 1989. A Phylogenetic analysis and reclassification of the Pococera-complex Genera (Lepidoptera: Pyralidae: Epipaschiinae) and a Preliminary Cladistic Analysis of the Pyralidae. Dissertation. University of Maryland, College Park. 428 p. [ Links ]
SOLIS , M. A. 1992. Check list of the Old World Epipaschiinae and the related New World genera Macalla and Epipaschia (Pyralidae). Journal of the Lepidopterists' Society 46 (4): 280-297. [ Links ]
SOLIS , M. A. 1993. A phylogenetic analysis and reclassification of the genera of the Pococera-Complex (Lepidoptera: Pyralidae: Epipaschiinae). Journal of the New YorkEntomological Society 101: 1-83. [ Links ]
SOLIS , M. A. 1995. Epipaschiinae, pp. 89-93. In: Heppner, J. B. (ed.). Check List Part 2: Atlas of Neotropical Lepidoptera, Brill/Flora and Fauna Books, Gainesville, FL. 243 p. [ Links ]
SOLIS , M. A. 1997. Snout moths: Unraveling the taxonomic diversity of a speciose group in the Neotropics, pp. 231-242. In: Reaka-Kudla, M. L.; Wilson, D.; Wilson, E. O. (eds.). Biodiversity II : Understanding and Protecting our Biological Resources. Joseph Henry Press, Washington, D. C. 551 p. [ Links ]
SOLIS , M. A. 1998. Michaelshaffera gen. n. - a pyraloid taxon lacking an abdominal tympanal organ (Lepidoptera: Pyralidae). Entomological Scandinavica 28: 391-402. [ Links ]
SOLIS , M. A. 1999a. Key to selected Pyraloidea (Lepidoptera) larvae intercepted at U.S. Ports of entry: Revision of Pyraloidea in "Keys to some frequently intercepted lepidopterous larvae" by D. M. Weisman, 1986. Proceedings of the Entomological Society of Washington 101 (3): 645-686. [ Links ]
SOLIS , M. A. 1999b. Nymphulinae Duponchel [1845] (Insecta: Lepidoptera): proposed precedence over Acentropinae Stephens, 1836. Case 3048. Bulletin of Zoological Nomenclature 56 (1): 31-33. [ Links ]
SOLIS , M. A. 2004. Systematics of Mexican stalkboring crambine Pyraloidea, pp. 6- 22. In: Rodríguez del Bosque, L.A.; Vejar Cota, G.; Cortez Mondaca, E. (eds.). Taller Internacional sobre Barrenadores del Tallo de Caña de Azúcar, Los Mochis, Sinaloa, México. Sociedad Mexicana de Control Biologico. 104 p. [ Links ]
SOLIS , M. A. In press. Aquatic Lepidoptera. In: Merritt, R. W.; Cummins, K. W. (eds.). Aquatic Insects of North America, Kendall/Hunt Publishing Company. [ Links ]
SOLIS , M. A.; ADAMS KI, D. 1998. Review of the Costa Rican Glaphyriinae (Lepidoptera: Pyraloidea: Crambidae). Journal of the New York Entomological Society 106 (1): 1-55. [ Links ]
SOLIS , M. A.; MA ES, K. V. N. 2002. Preliminary phylogenetic analysis of the subfamilies of Crambidae (Pyraloidea: Lepidoptera). Belgian Journal of Entomology 4: 53-95. [ Links ]
SOLIS , M. A.; MI TTER, C. 1992. Review and preliminary phylogenetic analysis of the subfamilies of the Pyralidae (sensu stricto) (Lepidoptera: Pyraloidea). Systematic Entomology 17: 79-90. [ Links ]
SOLIS , M. A.; SHAFFER, M. 1995. Pyralinae, pp. 80-81. In: J.B. Heppner, ed. Check List Part 2: Atlas of Neotropical Lepidoptera, Brill/Flora and Fauna Books, Gainesville, FL. 243 p. [ Links ]
SOLIS , M. A.; SHAFFER, M. 1999. Contribution towards the study of the Pyralinae (Pyralidae): historical review, morphology, and nomenclature. Journal of the Lepidopterists' Society 53 (1): 1-10. [ Links ]
SOLIS , M. A.; MUNROE, E.; BECKER, V. O. 1995. Chrysauginae, pp. 81-88. In: Heppner, J. B. (ed.). Check List Part 2: Atlas of Neotropical Lepidoptera, Brill/Flora and Fauna Books, Gainesville, FL. 243 p. [ Links ]
SOLIS , M.A.; HIGHT, S. D.; GORDON, D. R. 2004a. Alert: Tracking the cactus moth as it flys and eats it way westward in the U.S. News of the Lepidopterists' Society 46 (1): 3-4. [ Links ]
SOLIS , M. A.; YEN, S.-H.; GOO LSBY, J. R. 2004b. Description and life history of Lygomusotima New Genus, and Neomusotima conspurcatalis (Lepidoptera: Crambidae) from Australia and Southeast Asia feeding on Lygodium microphyllum. Annals entomological Society of America 97 (1): 64-76. [ Links ]
SOLIS , M. A.; DAVIS , D. R.; NIS HIDA, K. 2005. Biology and systematics of Albusambia elaphaglossumae, a new genus and species of Crambidae (Lepidoptera: Pyraloidea: Crambidae) mining the fronds of Elaphoglossum conspersum (Pteridophyta: Lomariopsidaceae) in Costa Rica. International Journal of Tropical Biology 53 (3-4): 487-501. [ Links ]
SPEIDEL, W.; MEY, W. 1999. Catalog of the Oriental Acentropinae (Lepidoptera, Crambidae). Tijdschrift voor Entomologie 142: 125-142. [ Links ]
SUTRISNO, H. 2002a. Cladistic analysis of the australian Glyphodes Guenée and allied genera (Lepidoptera: Crambidae: Spilomelinae). Entomological Science 5 (4): 457-467. [ Links ]
SUTRISNO, H. 2002b. A preliminary study on relationships among selected Australian members of the Tribe Spilomelini (Lepidoptera: Crambidae: Pyraustinae). Zoological Science 19: 915-929. [ Links ]
SUTRISNO, H; AZUMA , N.; HIGAS HI ,S .2006. Molecular phylogeny of the Indo-Australia Glyphodes and its allied genera (Insecta: Lepidoptera: Crambidae: Spilomelinae) inferred from mitochondrial COI and COI and nuclear EF- 1 a gene sequences. Species Diversity 11: 57-69. [ Links ]
YEN, S. H. 2004. Insecta: Lepidoptera, Crambidae, Acentropinae, pp. 545-554. In: Yule, C. M.; Sen, Y. H. (eds.). Freshwater invertebrates of the Malaysian Region. Academy of Sciences Malaysia. Kuala Lumpur, Malaysia. 861 p. [ Links ]
YEN, S. H.; SO LIS , M. A.; GOO LSBY, J. A. 2004. Austromusotima, a new musotimine genus (Lepidoptera: Crambidae) feeding on Old World climbing fern. Annals of the Entomological Society of America 97 (3): 397-410. [ Links ]
YOS HIYAS U, Y. 1985. A systematic study of the Nymphulinae and the Musotiminae of Japan (Lepidoptera: Pyralidae). Scientific Report of Kyoto Prefectural University 37: 1-162. [ Links ]
YOU, P.; LI, H.-H.; CHEN, K-L. 2002. Catalogue of the Nymphulinae and Musotiminae of China (Lepidoptera: Crambidae). Fauna of China 4: 37-64. [ Links ]
WAAGE, J. K.; MO NTGOM ERY, G. G. 1976. Cryptoses choloepi: A coprophagous moth that lives on a sloth. Science 193: 157-158. [ Links ]
WHALLEY, P. E. S. 1964. Catalogue of the Galleriinae (Lepidoptera, Pyralidae) with descriptions of new genera and species. Acta Zoologica Cracoviensia 9 (10): 561-617. [ Links ]
WHALLEY, P. E. S. 1970. A synonymic catalogue of the genera of the Phycitinae of the world. Bulletin of the British Museum (Natural History) Entomology 25: 33-72. [ Links ]
Recibido: 17-abr-2007 - Aceptado: 4-may-2007














