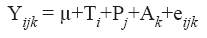Introduction
Two groups of polyunsaturated fatty acids (PUFA), linolenic (C18:3) and linoleic fatty acids (FA) (C18:2) have been recognized as very important for animal nutrition and human health. Flaxseed (Linum usitatissimum) has high concentrations of antioxidants and alfa-linolenic FA (C18:3n3), this PUFA represents approximately 53% of the total FA present in flaxseed (Petit and Côrtes, 2010). PUFA-rich seeds have been fed to dairy cows to improve milk fat quality by increasing its PUFA concentrations, mainly omega 3 FA. However, ruminal biohydrogenation transforms PUFA into saturated FA (SFA). Ruminal biohydrogenation is not entirely bad for ruminant products; some intermediates in this process have been shown beneficial for human health, such as C18:2 c9 t11 CLA (Shingfield et al., 2010). Therefore, it is necessary to control ruminal biohydrogenation to improve milk fat quality. The main FA of CLA family, C18:2 c9 t11, is the first intermediate of the ruminal biohydrogenation of C18:2, released by Butyrivibrio fibrisolvens. Furthermore, CLA can be synthesized in the mammary gland from C18:1 t11, another intermediate of incomplete biohydrogenation of unsaturated FA (Shingfield et al., 2010; Crumb and Vattem, 2011). According to several studies, these FA are beneficial for human health because they have anticarcinogenic and antiatherogenic properties, and improve immune response (Shingfield et al., 2010; Crumb and Vattem, 2011).
Heat treatment, such as extrusion and pelleting, has positive effects, by reducing the extent of biohydrogenation and preserving some PUFA (Gómez-Cortés et al., 2009; Neves et al., 2009; Dos Santos et al., 2011). Further, ionophores can transform milk FA; monensin increases C18:1 concentration and reduces C10, C12, C16, and 18:0 (FA of the end of biohydrogenation). Consequently, higher amounts of CLA may be formed in the rumen or in the mammary gland (Da Silva et al., 2007; Da Silva-Kazama et al., 2010). The aim of the present study was to evaluate the effects of pelleting and monensin addition on feed intake, digestibility of DM and nutrients, milk production, milk composition, and milk fat profile in Holstein cows kept on pasture and supplemented with concentrate containing ground flaxseed.
Materials and methods
Ethical considerations
All animal procedures were approved by the Animal Care and Use Committee of Universidade Estadual de Maringá (UEM), Brazil (Protocol No. 021/2008 - 036/2008).
Animals and diets
Four primiparous Holstein cows averaging 186 ± 10 d in milk and 515 ± 36 Kg body weight at the beginning of the experiment were used in a Latin square design with a 2×2 factorial arrangement of treatments and four 21-d periods (16 d for acclimation and 5 d for collections). The animals were kept on rotational grazing with free access to water. Cows were milked twice daily (06:00 and 15:00 h), and were subsequently housed in a tie stall for approximately 3 h to receive the concentrate feed. All diets contained ground flaxseed. The difference between treatments was the addition of sodium monensin and the pelleted vs no monensin addition and non-pelleted concentrate feed (Table 1). The concentrate feeds were balanced to provide isonitrogenous and isocaloric diets to meet the requirements of Holstein cows, according to NRC (2001). The pasture area consisted of four paddocks (0.4 Ha each) with Cynodon grass. Animals were placed in the paddocks when the grass was 40 cm high on average, and removed from them when the grass was about 20 cm high (Corrêa and Santos, 2003; Paris et al., 2016).
Table 1 Ingredients and chemical composition of concentrate feeds.

1GF: Concentrate with ground flaxseed. 2Ca = 156 g/Kg; P = 51 g/Kg; S = 20 g/Kg; Na = 93 g/Kg; K = 28 g/Kg; Mg = 33 g/Kg; Fe = 2,000 mg/Kg; Cu = 400 mg/Kg; Co = 30 mg/Kg; Cr = 10 mg/Kg; I = 40 mg/Kg; Se = 15 mg/Kg; Zn = 1,700 mg/Kg; F = maximum 510 mg/Kg; Mn = 1,350 mg/Kg; Vit. A = 135,000 IU/Kg; Vit. D = 68,000 IU/Kg; Vit. E = 450 mg/Kg. GFM: GF + monensin; GFP: GF pelleted; FMP: GF pelleted + monensin.
To determine pasture composition, samples of grazed stratum were manually collected from several points in each paddock. Grass contained on average 270 ± 15 g/Kg DM, 164 ± 25 g/Kg crude protein (CP), 22 ± 3 g/Kg ether extract (EE), 665 ± 46 g/Kg neutral detergent fiber (NDF), 315 ± 26 g/Kg acid detergent fiber (ADF), and 28 ± 1 g/Kg lignin. The available forage mass was 1.7 ton DM/Ha.
Experimental procedures and chemical analysis
The experiment was conducted during the wet season (December to February). Average precipitation and temperature were 175 mm and 26 °C, respectively during the trial (Maringá Main Climatological Station, located at UEM).
To estimate dry matter intake (DMI) and nutrient intake of concentrate feeds, on the last five days of each experimental period, concentrate and orts were sampled and stored at -20 oC for further analysis of DM, CP, EE, NDF and ADF. Roughage intake was estimated using indigestible dry matter (iDM) as internal marker, based on Cochran et al. (1986) and Casali et al. (2008). Indigestible DM was estimated in the samples of concentrates, orts, and feces by in situ digestibility during 240 h. Fecal excretion was estimated with chromium oxide (Cr2O3) as external marker. The Cr2O3 was provided orally in cellulose bags, twice daily (5 g for each animal), from the 5th to the 15th day of each experimental period (Detmann et al. 2001). Fecal samples were collected twice daily (08:00 and 16:00 h) from the rectum, between the 10th and 15th day, and a composite sample was proportionally collected on a dry weight basis per animal/period for further analyses. Samples of roughage, concentrate, orts, and feces were dried in a forced oven at 55 °C for 72 h and ground in a mill through a 1 mm screen to determine DM, CP, EE, NDF, ADF, and lignin.
Milk production was recorded daily and fatcorrected milk (FCM; 3.5%) was calculated as follows: FCM = (0.432 + 0.1625 × percentage of milk fat) x Kg milk/d (Sklan et al., 1992). Milk was sampled on the 17th and 18th day of each experimental period, and a composite sample was proportionately collected in relation to milk production. The sample was then stored in a plastic bottle and preserved with 2-bromo 2-nitropropano 1-3-diol (bromopol) for the analyses of dry extract, CP, fat, somatic cell count (SCC), and urea. A portion of the composite sample was stored at -20 °C for lipid composition analysis. Milk composition was determined through a Bentley-2000 infrared analyzer and SCC was determined using a Somacount-500. Milk fat was extracted by centrifugation (17,800 × g) for determination of FA methyl esters. Transesterification of triglycerides to obtain FA methyl esters was performed according to the 5509 method of ISO (1978) in n-heptane and KOH/methanol solution. The FA methyl esters were analyzed by gas chromatography in a Trace-GC Ultra equipment (Thermo Scientific, Tewksbury, MA, USA) equipped with automatic sampler, flame ionization detector, and a fused silica capillary column (100 m long, 0.25 mm internal diameter, and 0.20 μm - Restek Rt-2560 phase 100% bicianopropil polysiloxane). Gas output was 1.4 mL/min. Flow for the carrier gas (H2) and for the auxiliary gas (N2) was 35 mL/min, and 350 mL/min for synthetic air. Initial temperature of the column was 65 °C for 4 min, and then raised at a rate of 16 °C/min up to 185 °C, which lasted 12 min; then, at a rate of 20 °C/min. Final temperature of 285 °C was reached and maintained for 14 min. The injection volume was 1 microliter and the sample split ratio was 1:100. The peaks were identified through comparisons with the retention times of the standard FA methyl esters (Sigma, St. Louis, MO, USA).
Statistical analysis
Statistical analyses were performed with the PROC MIXED procedure [SAS, 2002. Statistical Analysis System, SAS/STAT® user’s guide. Version 9.0 (TS Level 00M0) Cary NC, USA] using a 2×2 factorial arrangement. The model was as follows:
Where:
Y ijk : Observation of k replication for i treatment in the j period.
μ: General mean.
T i : i treatment effect.
P j : j period effect (1, 2, 3, and 4).
A k : Animal effect (1, 2, 3, and 4).
e ijk : Random error associated with k animal receiving i treatment in the j period.
Treatments were compared to provide factorial contrasts: Pelleted vs non pelleted, with monensin vs without monensin, and the interaction between pelleting and monensin. We set the significance level at 5% (p<0.05), and up to 10% (p>0.051<0.10) as a tendency.
Results
Pelleting and monensin did not change milk production and composition (Table 2). Average milk production and milk fat concentration was 11.44 Kg/d and 37 g/Kg, respectively. Milk fat was reduced by monensin supplementation by approximately 8%. Proteins, lactose, total solids, and urea nitrogen content averaged 33.5, 45, 12.5 g/Kg, and 11.2 mg/dL, respectively.
The concentration of 16:1n9, 18:1 t9, 18:2n6, and CLA (C18:2 c9 t11) increased by the pelleting process (Table 3). Indeed, pelleting reduced C18, C20:2, C24 and milk SFA concentration. The concentration of C14 and C20 tended to decrease with the pelleting process. Monensin was effective for increasing C18:1 t9 and CLA content. Stearic acid (C18) was reduced by approximately 18% in the milk of cows fed pelleted diets. The CLA concentration in milk fat increased by approximately 47% by monensin addition. The CLA concentration was 70% higher in pelleted diets compared to non-pelleted diets.
The addition of monensin did not change PUFA, MUFA, SFA, ω6, ω3, PUFA/SFA, and ω6/ω3 ratios. Pelleting increased PUFA concentration by 25% and MUFA by 15%. Furthermore, milk SFA concentration was reduced from 55.17 to 47.38 g/100 g FA by pelleting, corresponding to a 14% reduction. Pelleted diets also increased ω6 concentration by 24.5% without changing the ω3 concentration in milk. In fact, PUFA/SFA and ω6/ω3 were 53.8 and 27.8% higher for pelleted diets, respectively. Therefore, the main effect on milk fat composition was observed with pelleted diets.
Nutrient intake was not affected by treatment (Table 4), with the exception of EE intake, where pelleting reduced intake by 28%. Considering the amount of monensin added to the concentrate feed (32.93 mg/Kg MS) and the DMI from concentrate feed (5.4 Kg/d), daily monensin intake was 178 mg/animal. This amount did not change the diet intake of cows. There were no differences on apparent total digestibility of nutrients because of pelleting and monensin.
Table 2 Production and milk composition of Holstein cows grazing on Cynodon pasture and supplemented with concentrate feed containing flaxseed.
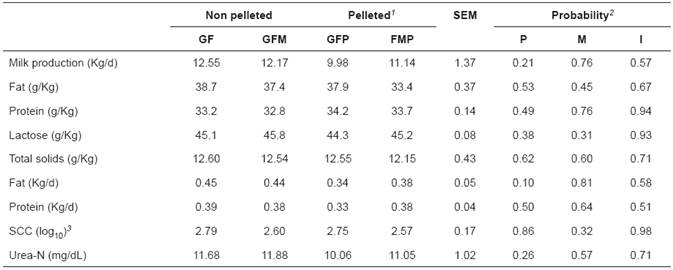
1GF: Concentrate with ground flaxseeds. 2SEM: Standard error of the mean. 3SCC: Somatic cell count. GFM: GF + monensin; GFP: GF pelleted; FMP: GF pelleted + monensin; P: Pelleting effect; M: Monensin effect; I: Interaction effect.
Table 3 Lipid composition (g/100 g of FA) of milk from Holstein cows grazing on Cynodon pasture and supplemented with concentrate feed containing flaxseed.

1GF: Concentrate with ground flaxseeds. 2SEM: Standard error of the mean. 3PUFA: Polyunsaturated FA; MUFA: Monounsaturated FA; SFA: Saturated FA. GFM: GF + monensin; GFP: GF pelleted; FMP: GF pelleted + monensin; P: Pelleting effect; M: Monensin effect; I: Interaction effect.
Table 4 Intake and total apparent digestibility of nutrients by Holstein cows grazing on Cynodon pasture and supplemented with concentrate feed containing flaxseed.

1GF: Concentrate with ground flaxseeds. 2SEM: Standard error of the mean. GFM: GF + monensin. GFP: GF pelleted; FMP: GF pelleted + monensin. P: Pelleting effect; M: Monensin effect; I: Interaction effect.
Discussion
Monensin has a limited effect on DMI (Ipharraguerre and Clark 2003; Possatti et al., 2015). Since it has the potential to increase the supply of gluconeogenic precursors, monensin administration might increase hepatic synthesis of glucose, thereby improving the energy balance in dairy cows (Ipharraguerre and Clark, 2003). Phipps et al. (2000) administered doses of monensin (0, 150, 300, and 400 mg/d) and did not observe differences in DMI. Similar to the present study, previous works did not find effect of the thermal process in DMI (De Marchi et al., 2013; 2015). Blood parameters are health indicators that can show the nutritional status and can be used to diagnose metabolic disorders. However, in the present study, no influence of the treatments on blood parameters was observed (data not shown), and all results remained within the recommended ranges.
The absence of effects on milk production and composition and nutrient digestibility could be attributed to the small amount of monensin ingested daily by the cows. Previous studies showed that milk production and composition, as well as DMI, were not affected in cows fed flaxseed and receiving 297 to 320 mg monensin/d (Petit et al., 2009; Da Silva- Kazama et al., 2011). Monensin acts directly on gram-positive bacteria that are primarily acetate and methane producers, thus, reducing ruminal production of acetic acid (Bergen and Bates, 1984; Russell and Strobel, 1989). Since acetic acid is a precursor of short chain FAin the mammary gland (Chilliard et al., 2000), monensin can reduce milk fat concentration up to 8%. Several reports on monensin effects on milk production and milk fat content range from decreases in milk production and milk fat content (Da Silva et al., 2007) to increases in milk production and no effect on milk fat concentration (He et al., 2012; De Marchi et al., 2015). The monensin doses in those studies were different; therefore, we cannot claim that absence of effect in the present study was only a function of monensin amount. Indeed, the effects of monensin intake have shown to be less significant in cows fed roughage (Possatti et al., 2015).
Despite the fact that monensin and pelleting did not have an effect on milk fat, the treatments, mainly pelleted diet, changed the FA profile of milk fat. The intake of food rich in SFA has been related to occurrence of coronary diseases and atherogenic effects due to increase of LDL cholesterol levels (Wang et al., 2016). We observed satisfactory results as the pelleted diets decreased SFA C14, C18, C20, and C24 by approximately 21, 18.5, 14, and 33%, respectively.
According to some studies, C14 and C16 are the major SFA present in the human diets. They have been indicated as the main dietary factors that raise LDL cholesterol and total blood cholesterol, and also are positively associated with the incidence of Type 2 diabetes (Noakes et al., 1996; Calder, 2015; Mensink, 2016). Indeed, reduction of some SFAs can be related with PUFA content in the diet, since long FA chains, such as C18:3 present in flaxseed, are inhibitors of the mammary synthesis of short and medium FA chains (Chilliard et al., 2000; Ward et al., 2002). A reduction of C18:0 in milk content (approximately 18% in the present study) by pelleted diets was also observed by Dos Santos et al. (2011) and De Marchi et al. (2015), who reported a reduction of 17 and 15%, respectively. This FA is the final product of ruminal biohydrogenation, and its low concentration in milk could indicate that biohydrogenation was inhibited and maybe more intermediates of this process, such as CLA and C18:1 t11, were synthesized in the rumen and posteriorly transferred to the mammary gland. Based on the present results, we can suggest that pelleting was efficient in protecting PUFA from ruminal biohydrogenation.
A more pronounced effect of the treatments was observed on CLA concentration, once C18:2 c9 t11 was increased by approximately 47 and 70% by monensin and pelleted diets, respectively, with a greater amount of CLA when monensin was added in association with pelleting, similarly to the results observed by Gómez-Cortés et al. (2009) and Dos Santos et al. (2011).
Both pelleting and monensin act as inhibitors of PUFA biohydrogenation in the rumen (Fellner et al., 1997; Neves et al., 2009). Ionophores inhibit the growth of gram-positive bacteria, such as Butyrivibrio fibrisolvens, one of the main bacteria responsible for fatty acid hydrogenation, thus, PUFA conversion into SFA can be reduced. Furthermore, gram-positive bacteria produce hydrogen, and lower hydrogen availability may interfere the biohydrogenation process (Fellner et al., 1997). Heat treatments of oil seeds alter the protein matrix that surrounds fat droplets. The protein matrix protects PUFA from biohydrogenation by a reduction in rumen degradation of fatty acids (Chouinard et al., 1997). This way, intermediate products of biohydrogenation can be later incorporated into milk or further converted into CLA by the delta-9-desaturase enzyme in the mammary gland.
The pelleted diet reduced milk SFA by 14% and increased PUFA, MUFA, ω6, PUFA/SFA, and ω6/ω3 by 25, 16, 25, 50, and 28%, respectively. Despite the small amount of monensin in the diets in the present study, it reflects the reality of many Brazilian farms where dairy cows are kept on pasture and receive concentrate feed. Our results suggest that, even at low amounts, monensin is able to improve milk FA quality of grazing cows, mainly increasing C18:2 c9 t11 (CLA) concentration. Pelleting and monensin do not alter milk production and composition. Pelleting, more than monensin, can improve milk fat quality once CLA, PUFA, and MUFA concentrations are increased in milk fat.