Introduction
Methionine (Met) is the second or third- limiting aminoacid (AA) in conventional diets for growing pigs, which can lead to its deficiency, reduced availability for protein synthesis and limited growth (Bauchart-Thevret et al., 2009; NRC, 2012; Chen et al., 2014). The Met levels present in most dietary ingredients for pigs are insufficient; thus, it must be provided from a synthetic source (Santos et al., 2011). Additionally, bioavailability and bioefficacy of synthetic DL-Met isomers show inconsistent results in growth performance of pigs (Shen et al., 2014; Kong et al., 2016). These could be attributed to the fact that free synthetic AA are very sensitive to the acidic conditions of the stomach and are rapidly absorbed in the digestive tract (Sato et al., 1984, Stein et al., 2007), decreasing their metabolic efficiency.
Dietary inclusion of protected AA provides resistance to stomach conditions and allows for slow intestinal release, which could help to overcome the above-mentioned limitations (Piva et al., 2007). Protected AA utilization has been reported in ruminants, improving productive performance (Zanton et al., 2014). Protected lysine, in substitution for HCl·lysine, enhances the bioavailability of this AA in pig diets without negatively affecting growth performance and carcass characteristics (Prandini et al., 2013).
Supplementing Met to pig diets has been associated with positive effects on the immune system. (Opapeju et al., 2012; de Oliveira et al., 2015). Although pigs can tolerate some shortage or surplus of Met (Pena et al., 2008; Santos et al., 2011; Ying et al., 2015) its excess can affect growth performance and carcass characteristics, while low Met levels can increase fat deposition, reducing protein synthesis (Conde-Aguilera et al., 2014). Appropriate dietary concentration of Met depends on each analyzed variable (Zhang et al., 2013).
The objectives of this study were to determine the optimum level of Met in pig diets and to evaluate the effects of regular synthetic or protected DL-Met on growth performance, carcass characteristics and plasma urea nitrogen (PUN) concentration.
Materials and Methods
Ethical Considerations
The experimental procedures followed the standards for ethics, biosafety and animal well- being of the Official Mexican Standard (NOM- 062-ZOO, 1999) for the use of animals in experimentation.
Location
The experiment was conducted at the Swine Unit of the Experimental Farm of Colegio de Postgraduados, located in Montecillo, State of Mexico (98º 48' 27'' W and 19º 48' 23" N). The climate is temperate, semi-arid, with 15.8 °C average annual temperature, infrequent frosts, 663.7 mm average annual rainfall, and 2,250 m altitude (Estación Agro-Meteorológica, 2016).
Animals and experimental design
Forty-eight crossbred pigs (Yorkshire x Landrace x Duroc) terminal line fattening barrows and gilts (11.74±1.72 kg initial BW) were used in a completely randomized design. The treatments consisted in the dietary supplementation with four levels of Met from two types (protected and regular Met) during four growing stages: Nursery (0.48, 0.53, 0.58, and 0.63%), Grower (0.38, 0.43, 0.48, and 0.53%), Finishing I (0.31, 0.36, 0.41, and 0.46%) and Finishing II (0.23, 0.28, 0.33, and 0.38%). These levels were obtained by adding synthetic or protected Met to the control diet. The Met concentrations of synthetic Met fulfilled the dietary requirements of Met+Cys suggested by the NRC (2012). The protected Met used contained 85% encapsulated DL-methionine (Mepron® Evonik Industries, Germany).
Diets and animal handling
Basal diets contained sorghum-soybean meal and were supplemented with crystalline AA [L-lysine·HCl, DL-methionine (Evonik Industries, Parsippany, NJ, USA), L-threonine (Jefo Nutrition Inc, Saint-Hyacinthe, Québec, Canada) and L-tryptophan (CPB Aurum, México, DF)] to meet or exceed the nutritional requirement for each stage of growth (NRC, 2012; Table 1). Pigs were individually housed in 1.2×1.5 m pens, on concrete floor partially covered with plastic slats, and equipped with a single feeder and a nipple drinker. The animals were injected with a deworming and vitamin product (Endovet polivitaminado® 15 μl kg-1 of body weight) before the start of the study. Pens were cleaned and the health of pigs verified throughout the experimental period on a daily basis. Ventilation was provided with curtains. Feed and water were provided ad libitum.
Table 1 Ingredients and nutrient composition of experimental diets.

*50.7% lysine (Biolys® Evonik).
**Supplied by kg of feed: 5.0×106 IU vitamin A; 1.0×106 IU vitamin D3; 2.0×104 IU vitamin E; 2 g vitamin K3; 1 g tiamine; 5 g de rivoflavin; 2 g pyridoxine; 25 g niacin; 15 g D-calcium panthotenate; 3 g folic acid; 225 g choline chloride; 0.3 g antioxidant; 15 mg B12; 180 mg biotin (REKA® Lapisa Animal Nutrition).
*** Supplied by kg of feed: 0.2 g Se; 0.1 g Co; 0.3 g I; 10 g Cu; 100 g Zn; 100 g Fe; 100 g Mn (REKA® Lapisa Animal Nutrition).
Variables measured
The variables analyzed were: daily weight gain (DWG), daily feed intake (DFI) and feed:gain ratio (FGR). In addition, on the first and last day of the experiment, the backfat thickness (BT) and longissimus muscle area (LMA) were measured using real time ultrasound (Sonovet 600) with a 3.5 MHz transducer (Medison, Inc., Cypress, California, USA). These data, together with the initial (BWi) and final (BWf) body weight, were used to determine fat free lean gain (FFLG) and lean meat percentage (LMP) using the NPPC equations (Burson and Berg, 2001). At the last day of each stage, blood samples were taken from the vena cava in heparinized tubes (Vacutainer®), then set in ice until centrifuged at 2,500 g during 20 min (IEC Centra 8R, International Equipment Company, USA), to separate plasma from cell package. Plasma was transferred to polypropylene tubes and stored in a freezer (EUR251P7W Tappan, Electrolux Home Products North America, USA) at -20 °C until determination of PUN concentration by UV spectrophotometry (Spectrophotometer Cary 1E UV-vis, Varian, Australia; Chaney and Marbach, 1962).
Statistical analysis
The experimental design was completely randomized with a 4×2 factorial arrangement of treatments. Factors were: four levels and two types of Met (regular and protected). Six replicates per treatment were used. Shapiro-Wilk and Levene's tests were used to check normal distribution and homogeneity of variance for all variables. Data were analyzed with the GLM procedure, and Tukey's test (p≤0.10) was used to compare treatment means (SAS, 2011). Initial body weight was used as a covariate (p≤0.10)
Results
Table 2 presents the growth performance results of nursery pigs. No interaction was found between level and type of Met on growth performance (p>0.10). The FGR was reduced (p=0.10) with the highest level of Met (0.63%). The use of protected Met increased DFI, DWG, BWf and BT (p=0.07).
Grower pigs consuming the highest levels of Met showed reduced DFI (p=0.08; Table 3). A possible interaction was observed between level and type of Met for DFI (p=0.10). The FGR improved (p=0.05) when using 0.48- 0.53% Met, although the best response by the interaction between Met level and type was observed with 0.53% of protected Met (p=0.05). The highest DWG (p=0.10) was observed with 0.43% Met, regardless of Met type (p>0.10). The PUN was reduced with 0.48% Met (p=0.10). Protected Met reduced LMP (p=0.09) and increased BT (p=0.02).
Table 4 shows the results of Finishing I pigs. The lowest BT was observed with 0.41-0.46% regular Met (p=0.09). Protected Met increased DFI (p≤0.01), DWG (p=0.04), FFLG (p≤0.01), LMP (p=0.07), and LMA (p=0.02).
The results of Finishing II pig variables are shown in Table 5. Protected Met increased DFI (p=0.02). The DWG, BWf, and FFLG improved with 0.28% protected Met (p=0.02). PUN was reduced (p=0.02) using 0.33% Met, regardless of Met type.
Discussion
Protected Met increased DWG in nursery and finishing pigs, raised DFI in nursery and finishing pigs, improved FGR in grower pigs, increased BT in nursery and grower pigs, and improved carcass characteristics in Finishing I pigs. Prandini et al. (2013) reported better metabolic efficiency of protected AAs compared with traditional synthetic AAs in pig diets due to slower release and absorption rates, which allows for higher metabolic utilization. Protected Met could increase availability and absorption of this amino acid in pigs (Piva et al., 2007; Schwab and Orway, 2016) leading to better productive performance, including DWG, final BW, and FGR (Opapeju et al., 2012; Shen et al., 2014; Chen et al., 2014). In the present study, the increased digestibility of protected Met possibly led to increased BT evidencing a possible excess and imbalance among AAs, which results in removal and excretion of nitrogen excess and retention of carbon skeletons as fat (García et al., 2010).
Table 2 Treatment means for growth performance of nursery pigs fed four levels and two types of methionine.

Met, Methionine; DFI, daily feed intake; DWG, daily weight gain; FGR, feed:gain ratio; BWi, initial body weight; BWf, final body weight; FFLG, fat free lean gain; LMP, lean meat percentage; BT, backfat thickness; LMA, Longissimus muscle area; PUN, plasma urea nitrogen concentration; SEM, standard error of the mean. Means with different superscript letters (a, b, c, d) within columns differ statistically (p≤0.10).
In our experiment, an extra 0.10-0.15% Met was the best level for FGR in nursery and grower pigs. Supplementation with extra 0.02- 0.12% Met has been reported to improve FGR in postweaning and growing pigs (Opapeju et al., 2012; Chen et al., 2014; de Oliveira et al., 2015). This could be explained by met being a functional AA for intestinal growth and function, beyond its role as a precursor for protein synthesis (Wu et al., 2013). In grower pigs, although DFI was reduced with 0.48 and 0.53% Met, FGR was improved with these levels.
Grower and Finishing II Pigs fed extra 0.05% Met resulted in higher DWG. In growing pigs, 0.01-0.15% additional Met reduced DFI, thus improving FGR. In Finishing I pigs, 0.10-0.15% extra Met reduced BT. In Finishing II pigs, the addition of 0.05% Met improved FFLG. The Met requirement found in this study is higher than that recommended by the NRC (2012). A higher Met level could represent extra benefits in terms of productive performance. Methionine supplementation (0.02, 0.04, and 0.06%) in growing pig diets has resulted in improved daily gain, feed intake (Opapeju et al., 2012), and feed: gain ratio (Opapeju et al., 2012; de Oliveira et al., 2015). On the contrary, a Met deficiency in pigs can lead to lower DWG (Conde-Aguilera et al., 2014), growth suppression of the intestinal mucosa, reduction of intestinal epithelium, and increase of oxidative stress (Bauchart-Thevret et al., 2009; Chen et al., 2014). Several studies have showed that pigs can tolerate some Met deficiency (Santos et al., 2011; Ying et al., 2015) or excess levels in the diet (Pena et al., 2008).
Table 3 Treatment means for growth performance of grower pigs fed four levels and two types of methionine.
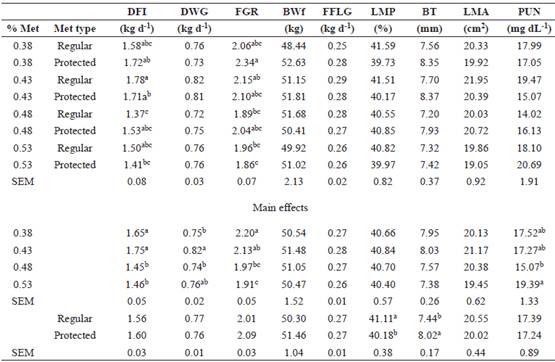
Met, Methionine; DFI, daily feed intake; DWG, daily weight gain; FGR, feed:gain ratio; BWi, initial body weight; BWf, final body weight; FFLG, fat free lean gain; LMP, lean meat percentage; BT, backfat thickness; LMA, Longissimus muscle area; PUN, plasma urea nitrogen concentration; SEM, standard error of the mean. Means with different superscript letters (a, b, c, d) within columns differ statistically (p≤0.10).
It has been also suggested that Met deficiencies could not affect growth performance, carcass characteristics and protein synthesis due to an adaptation of tissue metabolism when facing insufficient dietary met supply (Castellano et al., 2015). The results of the present study confirm that increasing Met levels improve growth performance variables in pigs. Protein deposition is affected when Met is provided at insufficient levels (de Oliveira et al., 2015); therefore, supplementation of extra Met (+0.12%) is required for optimal protein synthesis (Chen et al., 2014). Additionally, proper Met levels might reduce carcass fat content in pigs with Met deficiency (Conde- Aguilera et al., 2014; Castellano et al., 2015). In our study, adding extra 0.10% Met in growing and Finishing II pigs reduced PUN. This lower PUN concentration is associated with a lower synthesis and excretion of urea originated by AA excess, indicating better utilization of metabolic nitrogen (Qin et al., 2015).
In conclusion, substituting synthetic Met with protected Met increases DWG and DFI without negatively affecting FGR. Additionally, protected Met could alter lipid metabolism, considering it increases BT. These results indicate that increasing 0.05-0.15% Met improves FGR, DWG, FFLG, and reduces BT and PUN; potentially reducing the excretion of nitrogen in feces and urine to the environment.
Table 4 Treatment means for growth performance of Finishing I pigs fed four levels and two types of methionine.

Met, Methionine; DFI, daily feed intake; DWG, daily weight gain; FGR, feed:gain ratio; BWi, initial body weight; BWf, final body weight; FFLG, fat free lean gain; LMP, lean meat percentage; BT, backfat thickness; LMA, Longissimus muscle area; PUN, plasma urea nitrogen concentration; SEM, standard error of the mean. Means with different superscript letters (a, b, c, d) within columns differ statistically (p≤0.10).
Table 5 Treatment means for growth performance of Finishing II pigs fed four levels and two types of methionine.

Met, Methionine; DFI, daily feed intake; DWG, daily weight gain; FGR, feed:gain ratio; BWi, initial body weight; BWf, final body weight; FFLG, fat free lean gain; LMP, lean meat percentage; BT, backfat thickness; LMA, Longissimus muscle area; PUN, plasma urea nitrogen concentration; SEM, standard error of the mean. Means with different superscript letters (a, b, c, d) within columns differ statistically (p≤0.10).














