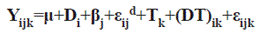Introduction
Cattle production is a central activity in Cesar (Colombia, South America), providing socioeconomical services, particularly to small producers. With over 1.4 million cattle, this province holds 6% of the inventory and 4.6% of the national dairy production (Colombian Agricultural Institute [ICA], 2020; Ministry of Agriculture and Rural Development of Colombia [MADR], 2018). Due to market globalization this sector faces serious environmental, social, and commercial challenges. Cattle production in this region requires transformation into a profitable, competitive, carbon neutral and ecologically sustainable activity to guarantee internal food safety and economic growth.
A limitation for this transition is related to animal feeding, given that production relies on grass monoculture (Mojica et al., 2017) with low production, availability, and nutritional quality of forages, mostly during the dry seasons. In consequence, the system poorly adjusts to the nutritional requirements of cattle, thus affecting its productivity and profitability (Murillo et al., 2014; Arce et al., 2013). The low nutritional quality and digestibility of forages, given their high content of structural carbohydrates and low protein, increases ruminal methane (CH4) emissions (Archimède et al., 2011). The CH4 has high global warming potential, which is 28 times higher than CO2 (IPCC, 2014) and represents from 2 to 12% of energy loss (Johnson and Johnson, 1995). Additionally, pasture expansion for cattle grazing has been associated with negative pressure over strategic ecosystems, causing deforestation, biodiversity loss, water contamination, soil compaction and other negative impacts on the environment (Cajas-Girón et al., 2012; Roncallo et al., 2012), with greenhouse gas (GHG) emissions being one of the biggest problems (Herrero et al., 2016).
Given this scenario of the cattle role on GHG emissions, mitigation strategies are required. Silvopastoral systems (SPS) are considered an alternative strategy for ruminant production that has positive effects on methane reduction (Galindo et al., 2016; Rivera et al., 2015). According to several researchers, the inclusion of legume forages in grass-based diets (such as Leucaena leucocephala, clover (Trifolium sp.) and other local forage leguminous resources) has a positive effect on methane reduction, ranging from 7 to 54% lower CH4 emissions (Molina et al., 2013; Navarro-Villa et al., 2011; Klein and Eckard 2008; Possenti et al., 2008).
The objective of this study was to evaluate the effect of tropical forage species in silvopastoral arrangements on methane production and fermentation parameters using an in vitro ruminal simulation technique - RUSITEC.
Materials and Methods
Ethical considerations
The Animal Care and Use Committee of Universidad Nacional de Colombia (CICUA-013) approved the use, handling and treatments of animals in the study.
Forage sampling
The forage grass and shrub forage samples were collected from pastoral monocultures and silvopastoral arrangements established by AGROSAVIA (Corporación Colombiana de Investigación Agropecuaria) at its Motilonia Research Center (Agustín Codazzi, Cesar, Colombia; 10°00’07’’N, 73°14’51’’W; 160 masl).
Annual average temperature is 27.8 °C, 70 % relative humidity, and 1,360 mm average rainfall. Sampling was performed during the dry season (April) using the hand-pluck method (Euclides et al., 1992) up to the regular grazing height (45 days regrowth). After collection, samples were analyzed in the Ruminal Biotechnology Laboratory (BIORUM) at Universidad Nacional de Colombia (Medellín, Colombia).
Treatments
Each diet was formulated based on the estimated dry matter consumption of animals grazing on monocultures and SPS (Cuartas et al., 2015) (Table 1). Treatments evaluated were: COL: 100% colosuana (Bothriochloa pertusa), CL: 70% colosuana + 30% Leucaena (Leucaena leucocephala), CG: 70% colosuana + 30% guácimo (Guazuma ulmifolia), CT: 70% colosuana + 30% totumo (Crescentia cujete).
Table 1 Composition of the four evaluated treatments.
| Treatment | Colosuana | Leucaena | Guácimo | Totumo |
| Inclusion (%) | ||||
| COL | 100 | - | - | - |
| CL | 70 | 30 | - | - |
| CG | 70 | - | 30 | - |
| CT | 70 | - | - | 30 |
COL: 100% colosuana (Bothriochloa pertusa), CL: 70% colosuana + 30% Leucaena (Leucaena leucocephala), CG: 70% colosuana+ 30% guácimo (Guazuma ulmifolia), CT: 70% colosuana + 30% totumo (Crescentia cujete).
Analyses of nutritional quality
Samples were dried using a forced air stove at 60°C for 48 hours at constant mass.
Crude Protein (CP) content was determined using the Kjeldhal method (AOAC, 2010). Neutral-detergent fiber (NDF) and acid-detergent fiber (ADF) were estimated using the methodology proposed by Van Soest et al. (1991); lignine content (LIG) was determined using sulfuric acid (72% v/v) on the ADF residue. Ash content (AC) was measured by direct incineration of the sample in a muffle at 500°C for 4 hours (adapted from AOAC 942.05), and organic matter (OM) content was calculated as the difference between DM and AC. Ethereal extract (EE) was determined with the Soxhlet method (AOAC, 2010).
In vitro ruminal simulation technique RUSITEC
For the in vitro fermentation, a semicontinuous RUSITEC system (Rumen simulation technique) was used following Czerkawski and Breckenridge (1977).
The ruminal content (liquid and solid) for the initial inoculum was obtained from two Holstein breed cows (700 ± 25 kg live weight) equipped with permanent rumen cannula, adapted to a diet based on angleton grass (Dichantium aristatum) hay; with CP, NDF, ADF and in vitro dry matter digestibility (IVDMD) values of 3.95, 78.5, 52.3, and 45.2%, respectively. Both cows were housed at Paysandú Experimental Farm, property of the National University of Colombia in Medellín (Colombia). The ruminal liquid was collected in thermos, preheated to 39 °C, to guarantee temperature and anaerobiosis conditions during transfer to the laboratory. Once in the laboratory, the liquid was mixed and filtered through two layers of muslin (0.45 mm pore size) under constant gassing with CO2. The RUSITEC system consisted of eight vessels (fermentation units) with 700 ml effective volume, kept in a 39 °C water-bath. To begin incubation, 80 grams (g) of the solid portion of the ruminal content and 12 g of the treatment to be evaluated were deposited in each fermenter into 100 μm pore size nylon bags, 500 mL of ruminal liquid, and 200 mL of pH = 8.3 artificial saliva (McDougall, 1948). After 24 h, the bag with the solid ruminal content was replaced in each fermenter by one with a new substrate (treatment). In the following days, each bag was replaced at 24-hour intervals, guaranteeing 48-hour incubation per bag. Daily pH was measured immediately after sample collection, using a pH meter (Metrohn model 704, Herisau, Switzerland). During the bag exchange process, the containers were gassed with pure CO2 to maintain anaerobic conditions. Throughout the experiment, saliva was prepared daily and was continuously supplied in each fermenter at a rate of 535 mL/d (2.47%/h) with a peristaltic pump and constant agitation through a mechanical platform (rate: 10-12 movements/ minute) to avoid dilution of the products resulting from the fermentation system. The volume of kitasatos flasks (Glassco, Haryana, India) was measured daily to guarantee the flow of artificial saliva in the fermenters.
During sampling, from days 7 to 13, the effluent liquid was collected in 1L kitasato flasks with a solution 20% v/v of H2SO4 (Merck, Whitehouse Station, NJ, USA) to determine VFA profile and ammonia, while the nylon bags with diet residue were used to determine dry matter degradability (DMD). The experiment was carried out in two periods (blocking factor). Incubation in each period lasted 10 days, with 7 days of adaptation (from day 0 to 7). The measurements were made from day 8 to day 10. The treatments were randomly assigned to the fermenters per period, with four replicates per treatment.
In vitro nutrient degradability, fermentation parameters and methane production
The DMD was calculated as the difference between incubated DM and degraded DM after 48 hours. To determine degradability of cell wall components, NDF and ADF percentage was determined from the residual DM following Van Soest et al. (1991). The NDF degradability (NDFD) and ADF degradability (ADFD) were estimated as the difference between incubated NDF or ADF, and degraded NDF or ADF, respectively. The OM digestibility (OMD) was determined considering the incubated OM and the degraded OM. Crude protein degradability (CPD) was determined after a previous detachment of ruminal microorganisms using a metil-cellulose solution (Whitehouse et al., 1994). The CP was then estimated using Kjeldhal’s method (AOAC, 2010). The CPD was obtained from the difference between incubated and degraded CP. Ammoniacal nitrogen (N-NH3) was quantified following protocols described by the AOAC (1999), using an ammonium selective electrode ISE.NH3-N (Metrhom model SM703, Herisau, Switzerland). Volatile fatty acids (VFA) were estimated using gas chromatography (GC) (Shimadzu model GC2014, Kyoto, Japan) equipped with autosampler, auto-injection and an Agilent® HP-FFAP polyethylene glycol capillary column of 25 m length x 0.32 mm internal diameter x 0.5 μm film thickness (Agilent Technologies©, Santa Clara, CA, USA). A flame ionization detector (FID) was used with helium as carrier gas at a constant speed of 42 cm/second. The temperature of the split injection port and detector were 260°C and 280°C, respectively. For quantification purposes, problematic samples were compared to the retention times of established standards.
The volume of gas produced was measured through a dry process gasometer (Shinagawa, Model DC-1C, Tokyo, Japan). The gas produced was collected in 5 L aluminium bags and a hermetic closure valve, which were connected to the kitasato flasks containing the effluent, while the samples for CH4 determination were collected in vacutainers. The CH4 analysis was performed using GC (Shimadzu, model GC2014, Kyoto, Japan) equipped with FID and an Agilent® HPPLOT Molesieve 5Å capillary column of 30 m length x 0.32 mm internal diameter x 12 μm film thickness (Agilent Technologies©, Santa Clara, CA, USA). The carrier gas used was UHP 5.0 grade helium at a linear speed of 35.4 cm/second. Injector and detector temperatures were 100°C and 300°C, respectively.
Statistical Analysis
The fermentation variables were subjected to analysis of variance (ANOVA) using the MIXED procedure of the SAS® program (version 9.1.3; SAS Institute Inc., Cary, NC, USA; 2001) through a randomized complete block (RCB) design with univariate structure and analysis of repeated measures in time (three measurement times) with 4 treatments, 8 repetitions and 2 measurement periods. The model included the fixed effects of diet, the measurement time, and their interaction. The experimental period (blocking factor) and the fermenter (experimental unit) were considered as random effects. Since there were no effects of measurement time or diet x time interactions, these data were not included in the tables. The covariance structures were adjusted by using mixed models with fixed and random effects, which allowed estimating the most appropriate standard errors for the different comparisons, according to the characteristics of each set of values. The minimum values of the Schwarz's Bayesian Information Criterion (BIC) were used to select the covariance structure.
Significant differences were compared using a Tukey test with a 5% significance level (p<0.05), and values between 0.05≤p≤ 0.10 were considered as trends.
The statistical model applied was:
Where,
Yijk: reading for the i-th treatment, on j-th block, at k-th time.
μ: overall population average
Di: i-th diet effect.
βj: j-th block effect (1 to 2).
εd: error between diets
Tk: measurement time (1 to 3).
(DT)ik: diet and measurement time interaction (1 to 12).
εijk: experimental error.
Results
Chemical composition and in vitro dry matter digestibility
The OM contents were between 88.61 and 89.33%. The CP content was the lowest for COL (7.85%), while inclusion of Leucaena leucocephala (CL), Guazuma ulmifolia (CG) and Crescentia cujete (CT) increased CP (41, 31 and 26%), respectively, compared to the control treatment (COL). Structural cell wall components, ADF and NDF, were higher for COL as compared to other treatments, while LIG was higher in CL, CG and CT, compared to COL (Table 2).
Regarding IVDMD, values were found between 42.43 and 49.05%, with higher digestibility in the three shrub diets (CL, CG and CT). For the forages used in this study, CP contents were between 12.6 for C. cujete and 28.4% for L. leucocephala, the latter standing out for its high protein content. The OM contents were between 90.8 and 92.4%, and AC were between 7.6 and 9.2%. The NDF contents (between 42.2 and 56.7%) were lower for shrub forage compared to grass-based diets of B. pertusa. The ADF content ranged from 24.7 to 36.5%, while LIG ranged from 15.5 to 17.8%, being higher on grass-based diets.
pH, Methane production and in vitro fermentation parameters
As shown in Table 3, the pH was not affected (p>0.05) by the inclusion of shrub forage in the diets.
Gas production significantly diminished (p<0.05) between 9 and 13% in diets with 30% inclusion of forage resources (CL, CG and CT) compared to the control treatment (COL), even when DMD increased between 5 and 9% and OMD not was affected for treatments CL, CG and CT compared to COL.
Table 2 Chemical composition and in vitro dry matter digestibility of the diets under evaluation.
| Diet | OM | AC | CP | NDF | ADF | EE | LIG | IVDMD |
|---|---|---|---|---|---|---|---|---|
| COL | 88.6 | 11.4 | 7.8 | 73.9 | 38.4 | 1.2 | 8.3 | 42.4 |
| CL | 89.6 | 10.4 | 13.1 | 58.1 | 33.2 | 1.5 | 9.3 | 47.9 |
| CG | 89.3 | 10.7 | 8.2 | 63.9 | 36.5 | 1.8 | 13.9 | 43.8 |
| CT | 88.8 | 11.2 | 10.5 | 62.7 | 33.9 | 2.0 | 9.5 | 49.1 |
| L | 92.4 | 7.6 | 28.4 | 42.2 | 24.7 | 2.7 | 15.5 | 49.0 |
| G | 91.6 | 8.4 | 14.6 | 52.2 | 33.7 | 1.9 | 17.8 | 39.7 |
| T | 90.8 | 9.2 | 12.6 | 56.7 | 36.5 | 1.8 | 15.7 | 44.6 |
COL: 100% colosuana (Bothriochloa pertusa), CL: 70% colosuana + 30% Leucaena (Leucaena leucocephala), CG: 70% colosuana + 30% guacimo (Guazuma ulmifolia), CT: 70% colosuana + 30% totumo (Crescentia cujete), L: Leucaena, G: guacimo, T: totumo. Chemical composition expressed as percentages of dry matter (DM), organic matter (OM), ash content (AC), crude protein (CP), neutral-detergent fiber (NDF), acid-detergent fiber (ADF), lignine (LIG), ethereal extract (EE), and in vitro dry matter digestibility (IVDMD). Different superscript letters ( a, b, c ) within columns indicate significant statistical difference according to the TukeyKramer test (p<0.05).
Table 3 In vitro ruminal fermentation parameters and nutrient degradation of four treatments after 48 hours of incubation in a semi-continuous ruminal simulation system (RUSITEC).
| Parameter | Diet | ||||
|---|---|---|---|---|---|
| COL | CL | CG | CT | p-value | |
| pH | 6.87 | 6.86 | 6.86 | 6.84 | 0.3763 |
| Total gas (L/day) | 2.38 a | 2.09 b | 2.17 b | 2.14 b | 0.0019 |
| N-NH3 (mg/dL) | 4.17 b | 7.50 a | 4.05 b | 4.20 b | <0.0001 |
| DMD (%) | 46.24 b | 50.27 a | 48.84 a | 48.36 a | 0.0040 |
| OMD (%) | 42.42 | 45.78 | 44.20 | 43.68 | 0.1165 |
| NDFD (%) | 34.39 a | 27.43 b | 32.30 a | 28.30 b | <0.0001 |
| ADFD (%) | 29.52 a | 29.55 a | 27.66 a | 23.10 b | <0.0001 |
| CPD (%) | 63.39 a | 64.82 a | 55.75 b | 47.83 c | <0.0001 |
| Total VFA (mmol/L) | 21.62 | 21.13 | 22.22 | 23.24 | 0.1754 |
| Acetate (mmol/L) | 14.08 | 13.77 | 13.50 | 13.80 | 0.1163 |
| Propionate (mmol/L) | 5.47 | 5.62 | 5.59 | 5.70 | 0.5733 |
| Butirate (mmol/L) | 1.71 | 1.68 | 1.67 | 1.98 | 0.2916 |
| Isobutyrate (mmol/L) | 0.10 | 0.11 | 0.10 | 0.10 | 0.1762 |
| Valerate (mmol/L) | 0.31 | 0.33 | 0.32 | 0.30 | 0.2824 |
| A:P | 2.45 | 2.43 | 2.41 | 2.42 | 0.1000 |
| CH4 (mL/day) | 12.61 a | 9.38 bc | 10.47 ac | 11.76 ac | 0.0300 |
| CH4 (mL/g DMi) | 1.23 a | 0.96 b | 0.95 b | 1.19 a | 0.0214 |
| CH4 (mL/g DMd) | 2.70 a | 1.94 bc | 2.02 ac | 2.30 ac | 0.0345 |
| CH4 (mL/ gNDFd) | 4.89 | 4.08 | 4.26 | 4.62 | 0.1355 |
| CH4 (mL/gADFd) | 10.92 a | 8.53 ac | 6.47 bc | 8.96 ac | <0.0001 |
| CH4 (mL/gOMd) | 3.17 a | 2.23 bc | 2.20 bc | 2.90 ac | 0.0035 |
COL: 100% colosuana (Bothriochloa pertusa), CL: 70% colosuana + 30% Leucaena (Leucaena leucocephala), CG: 70% colosuana + 30% guacimo (Guazuma ulmifolia), CT: 70% colosuana + 30% totumo (Crescentia cujete), DMD: dry matter digestibility, OMD: organic matter degradation, NDFD: neutral-detergent fiber digestibility, ADFD: acid-detergent fiber digestibility, CPD: crude protein digestibilty, pH: potential hydrogen, N-NH3: ammoniacal nitrogen, VFA: volatile fatty acids, A:P ratio: acetate:propionate ratio, Gas: gas production, CH4: methane, DMi: incubated dry matter, DMd: degraded dry matter, NDFd: degraded neutral detergent fiber, ADFd: degraded acid-detergent fiber, OMd: degraded organic matter. Different superscript letters ( a, b, c ) within columns indicate significant statistical difference according to the Tukey-Kramer test (p<0.05).
The NDFD significantly diminished (p<0.05) in CL (27.43%) and CT (28.3%) compared to the control (34.49%), but no difference was found with CG (32.3%). The ADF degradation only diminished in CT compared to other treatments. The CPD was higher (p<0.05) for COL (63.69%) and CL (64.82%) in comparison to CG (55.75%) and CT (47.83%). The N-NH3 concentration increased (p<0.05) by 44 to 46% in CL compared to others.
On the other hand, total VFA production and molar proportions of individual VFA were not affected (p>0.05) by forage inclusion. The acetate:propionate (A:P) ratio showed a tendency to decrease (p= 0.10) in diets CL, CG and CT compared to COL.
Methane production expressed in mL/d, mL/g DMd, mL/g DMi, and mL/g OMd, had similar emissions in all diets, showing a significant reduction (p<0.05) for CL compared to COL.
Discussion
Inclusion of shrub forage as a protein source in low quality diets represents an effective alternative to improve the nutritional quality of grass-based diets, increasing animal productivity, particularly in the low tropics (Argüello-Rangel et al., 2019). Additionally, secondary compounds from the plants used in ruminant feeding can be used to manipulate ruminal fermentation dynamics (Anantasook y Wanapat, 2012). Under the present conditions, DMD increased in all diets with inclusion of shrub forage, which could result in increased passage rate and voluntary consumption of forage (Choque et al., 2018). These results agree with Molina et al. (2015), who reported that 27% inclusion of L. leucocephala foliage in a grass-based diet increased DMD by 12%. The low degradability of fiber fractions (NDFD and ADFD) in diets with shrub forage could have resulted in lower cellulolytic activity due to the effect of secondary compounds, such as tannins and saponins, present in L. leucocephala (Soltan et al., 2017), G. ulmifolia (López et al., 2004) and C. cujete (Pereira et al., 2017) on ruminal microbial populations, particularly protozoa, responsible for up to 25-30% of fiber degradation (Lee et al., 2011), or through the formation of cellulose complexes, reducing carbohydrates degradation (Khiaosa et al., 2015).
The lowest (p<0.05) fiber digestibility was observed in the diet with L. leucocephala (NDFD) and C. cujete (NDFD and ADFD), possibly due to tannins and phenynins in different genres of this shrub, which form cellulose complexes and reduce carbohydrate degradation, limiting rumen degradation (Parente et al., 2016; Rojas et al., 2015; Khiaosa et al., 2015). The effect of these compounds may be due to union with sterols of the microbial membranes, particularly cholesterol, forming insoluble complexes and lysing the cells (Ramos-Morales et al., 2017). These compounds can also affect digestibility by binding to proteins and other nutrients, limiting microbial enzymatic activity -for example, α-glycosidase and α amylase (Li et al., 2011)thereby affecting fermentative processes in the rumen (Jayanegara et al., 2014).
In this context, it is suggested that decreased degradation of structural components may be due to adaptation of microbial populations to the new ruminal environment (Sampaio et al., 2009). According to Bodas et al. (2012), the effects of secondary metabolites are more evident in in vitro than in in vivo studies, probably because the compound is distributed more uniformly in batch or continuous cultures, and microbes are more quickly exposed to the activity of the phyto-chemical.
In this study, CPD was not affected in CL as compared to COL; however, CPD decreased (p<0.05) in CG and CT, which corresponds to the high CP content of this legume. However, the degradation percentage of this nutrient in all diets was greater than the degradability of the wall constituents (NDFD and ADFD), showing that secondary compounds, like tannins and saponins, bind with higher affinity to fiber (NDF and ADF) than to protein. In L. leucocephala, 0.16 and 0.22% of condensed tannins were bound to CP and fiber, respectively (La O et al., 2003). These differences may be associated with the type of tannins in the forages. Hydrolysable tannins do not generate a bypass effect as occurs with condensed tannins because the formers do not establish rigid bonds with proteins at pH range of the rumen (McSweeney et al., 1988).
These CP degradability in rumen suggest that the bypass protein would be approximately 40%, which is beneficial since it would improve the synthesis of microbial protein. This guarantees the flow of protein with high biological value to the small intestine (duodenum) favouring an efficient use of forages by ruminants (Rodríguez et al., 2010; Wickersham et al., 2008) and increasing milk protein (Martínez et al., 2004) as well as decreasing nitrogen and ammonia excretion to the environment (Cobellis et al., 2016).
The N-NH3 concentration increased (p<0.05) in CL compared to all other diets. This could be due to the higher CP content and CPD in this diet, supporting Javaid et al. (2008), who reported that increased degraded protein in the rumen raises ammoniacal nitrogen levels in buffalos. Other researchers have also reported a positive association between level of protein supplementation and ruminal ammonia concentrations (Mathis et al., 2000; Wickersham et al., 2008).
Therefore, the synchronization between N-NH3, CPD, and CP in the diet results in increased microbial protein synthesis (Chanthakhoun et al., 2012). In this regard, Detmann et al. (2009) found that the ammonium concentration in rumen where degradation and NDF consumption is optimized, was between 8 to 15 mg/dL. Fiber degradation is limited below that level. However, Slyter et al. (1979), evaluating the effect of ruminal ammonium concentration in steers receiving a forage diet with 8% CP, found that 2 to 5 mg/dL of N-NH3 in the ruminal fluid was sufficient to allow maximum growth of rumen microorganisms.
The low contents of ammoniacal nitrogen observed in treatments CG and CT may be explained by the moderate contents of condensed tannins (<4% DM) in Guazuma ulmifolia and Crescentia sp, (Rojas et al., 2015). Tannins bind with dietary proteins, inhibit microbial deaminases and reduce proteolysis, diminishing protein degradation in the rumen, consequently diminishing N-NH3 (Szumacher-Strabel and Cieślak, 2012; Bhatta et al., 2009; Min et al., 2006; Newbold et al., 2004). However, ammonium concentration in LC was higher compared to the other treatments, which is explained by the high CPD, due to a low affinity of tannins and saponins in Leucaena with the protein (La O et al., 2003).
The VFA are the main products of ruminal fermentation. They regulate physiological processes in ruminants, such as cholesterol, insulin, and glucagon synthesis (Mao et al., 2016). In the present study, total VFA concentration and individual VFA profiles were not affected by forage inclusion, supporting the pH stability among diets. However, the A:P ratio showed a tendency to diminish in diets supplemented with forages, possibly due to high DM degradation, also related to the reduction of methane synthesis given that propionate production requires hydrogen (Martin et al., 2010). Patra and Yu (2015) found reductions in the A:P ratio related to decreased protozoal populations, which are associated with methanogenic bacteria. This is a side effect of secondary compounds, given that acetate and butyrate are the main fermentation products of these microorganisms. As an outcome, it contributes to low methane production and hig microbial protein availability. Secondary compounds in L. leucocephala, G. ulmifolia and C. cujete could explain the VFA profiles obtained in the present study (Soltan et al., 2017; Galindo et al., 2014; Alvear et al., 2013). These compounds also inhibit growth of Gram-positive bacteria (generally acetate producers) favouring propionate-producing bacterial species through competition (Wallace et al., 2002).
Similarly, Apráez et al. (2012) found that inclusion of Acacia decurrens (forage with tannins) in Raygrass-based diets decreased acetate production by 54% and methane by 80% compared to an only-Raygrass diet. Variations in total gas and methane production among diets may be explained by the variations in their chemical composition.
The results from the present study are comparable to those of Anantasook and Wanapat (2012) who found total VFA between 22.1 and 34.8 mmol/L after including leguminous resources into the diet.
The diet with L. leucocephala had the strongest decrease in methane production, likely due to the presence of tannins and low contents of fiber in this leguminous resource (Soltan et al., 2017). The effects of tannins and saponins over methanogenesis have been demonstrated in vivo (Tiemann et al., 2008; Beauchemin et al., 2007) as well as in vitro (Jayanegara et al., 2011; Tavendale et al., 2005). The effect of these components is related to direct inhibition of methanogenic archaea and/or depression of microbial metabolic processes implied in methanogenesis (Li et al., 2014; Patra, 2012). However, an antiprotozoal effect has also been proved through complex formation with sterols in the plasmatic membranes of protozoa (Jouany and Morgavi 2007; Goel and Makkar 2012), consequently reducing methane production given their symbiotic relationship with methanogenic archaea (Kobayashi, 2010). Bodas et al. (2008) and Kamra et al. (2008) evaluated the potential effect of plant extracts on in vitro methanogenesis, reporting reductions in CH4 between 15 and 25% associated with decreased methanogenic populations without adverse effects on digestibility or VFA production.
From these results, a reduction of ruminal methane synthesis can be inferred, therefore lowering the emission of this greenhouse gas into the atmosphere, which is detrimental to the environment by contributing to global warming and it also represents a loss of energy for the animal (Martínez et al., 2017; Yang et al., 2015).
In conclusion, 30% dietary inclusion of forages such as Leucaena leucocephala, Guazuma ulmifolia and Crescentia cujete in grass-based diets of B. pertusa increased protein content and decreased fiber in the diets, improving DMD, ammoniacal nitrogen production, and the dynamics of ruminal fermentation parameters, while decreasing methane production. This suggest L. leucocephala, G. ulmifolia and C. cujete are valuable feed supplements to improve nutrient utilization efficiency in ruminants in the low tropics.