Introduction
Scabies is an important problem threatening health of humans and animals (Mcclain et al., 2009). It was first detected microscopically in 1687 by the Italian physician Giovan Cosimo Bonomo. Linnaeus identified two different agents of scabies, one in animals and the other in humans (Ljunggren et al., 2005). Sarcoptes scabiei var canis, Otodectes spp., trombiculid mites, Cheyletiella yasguri as well as Demodex canis, are the causative agents of mange in dogs in tropical and subtropical regions (Hampel et al., 2018). Among all these, the agents S. scabiei and D. canis have significant roles in the deterioration of health and welfare of infected animals (Paterson et al., 2014). Sarcoptes scabiei, with a worldwide distribution, affects especially young domestic dogs. Sarcoptic mange causes severe skin lesions that can affect dog skin, mostly in the form of alopecia, thickened skin structure, intense itching, dry, exudative crusts, and hemorrhagic and non-hemorrhagic cracks on the facial skin, upper neck, and upper eyelids (Uzuegbu et al., 2015).
Pro-inflammatory cytokines such as Interleukin 1α, Interleukin 1β, Interferon-γ and Tumor necrosis factor-α have a remarkable role in the occurrence of the disease (Arlian et al., 1996; Lalli et al., 2004). Triggering of these cytokines leads to overproduction of reactive oxidants and free radicals including free reactive oxygen species (ROS) such as hydroperoxide radicals (OH), superoxide anion radicals (O2), and reactive nitrogen species such as nitric oxide (NO) (Bickers and Athar, 2006). Antioxidants have an important role in preventing cell damage by means of reducing free radicals (Kleczkowski et al., 2003). Healthy animals keep a balance between the antioxidant system and free oxygen radicals, and the change of the balance is known as oxidative stress (Ercan and Fidanci, 2012). In addition, when excessive ROS accumulation occurs along with insufficient antioxidant mechanism to neutralize free radicals the cellular function may change and biological damage may occur (Knight et al., 2000). Free radicals also cause adverse effects such as inflammation, edema, wrinkles, erythema, autoimmune reaction, keratinization abnormalities, and hypersensitivity on the skin. The tunneling activity in the skin caused by parasites in scabies is an important cause of oxidative stress (Camkerten et al., 2009).
Few studies have investigated oxidative stress in sarcoptic mange (De and Dey, 2010; Allaam et al., 2014; Beigh et al., 2016). Additionally, no study has evaluated malondialdehyde (MDA), total antioxidant status (TAS), oxidative stress index (OSI), glutathione (GSH), NO, and total oxidant status (TOS) markers in dogs with sarcoptic mange as well as RBC, HGB and PCV counts. Consequently, the evaluation of oxidative stress by using MDA, TAS, TOS, GSH, NO, and OSI markers along with some hematological parameters in dogs naturally infected with S. scabiei was aimed in this study.
Materials and Methods
A total of 32 mixed-breed dogs between 1 and 2 years of age (Ethics Committee Decision No: 185/2021) were evaluated in the study. The dogs were allocated into 2 groups which were control group (infestation-free animals; n=10), and a sarcoptic mange group (Sarcoptes; n=22). The dogs in the Sarcoptes group were naturally infected with sarcoptic mange and they showed infestation signs such as intense itching, excoriations, alopecia, and blistering of the elbow and auricular margins.
Scrapings (scraping area varying from 1to5 cm) from the center and peripheries of the lesions on different parts of the face, ear, neck, elbow, shoulder, and tail of dogs diagnosed with clinical scabies was taken into sterile containers. The samples were mixed with 5 mL 10% KOH to be free of tissue materials, and then two drops were taken and put on a slide to examine under a microscope at 10X magnification (Shalaby et al., 2016).
Blood samples were taken from vena cephalica antebrachium into 10 mL serum tubes (Vacutainer tube with clot activator, Becton Dickinson Co. USA) and sterile test tubes containing 0.14% anticoagulant (EDTA K3, Pty Ltd., Adelaide, SA, Australia). The samples were kept at room temperature during 30 min. Then they were centrifuged at 3,000 rpm×10 min, and sera samples were stored at -80 °C until analyses. For complete blood cell count, an Abacus® Junior Vet5 (Diatron In. Budapest, Hungary) automated blood cell counter was used (WBC, RBC, HGB, and PCV).
Malondialdehyde levels of sera samples were determined spectrophotometrically by the method of Placer and colleagues with modification (Placer et al., 1966). The thiobarbituric acid (TBA) assay was used to measure MDA levels in sera samples. A pink color was observed in TBA and MDA reactions and then the color was analyzed at 532 nm in the spectrophotometer. The MDA levels are expressed as nmol/g blood.
The Sedlak and Lindsay method was used to measure GSH levels of blood. For this purpose, 5,5’-Dithiobis (2-nitrobenzoic) acid is reduced in this method by compounds containing sulphydryl groups and the acquired yellow color was assayed at 412 nm in the spectrophotometer. The GSH levels are expressed as nmol/g blood (Sedlak and Lindsay, 1968).
A novel automated method developed by Real Assay Diagnostic (Turkey) was used to determine sera TAS levels. The oxidative reactions initiated by hydroxyl radicals in the mixture are inhibited by the antioxidant components in the sample, and inhibition of the color change and TAS are measured in the sera sample (Erel, 2004).
The TOS levels in sera were determined by using a novel automated method (Erel, 2005). The ferrous ion-O-dianisidine complex is converted to ferric ion by oxidants in the plasma sample. The oxidation reaction is enhanced by glycerol molecules plentifully available in the reaction medium. The ferric ion in an acidic medium forms a colored complex with xylenol orange. Color intensity is measured by spectrophotometry.
The OSI value was obtained with the following formula:
OSI = TOS (μmol H2O2 equivalent/L)/TAS (μmol Trolox equivalent/L) x100.
Sera NO levels were determined with a commercial test kit (Enzo Life Science, USA). The sera NO measurements are based upon the enzymatic conversion of nitrate to nitrite and the colorimetric detection of nitrite, a colored azo dye compound of the Griess reaction.
The SPSS statistics program (Statistical Package for Social science) was used for statistical analysis. Since preliminary results of Kolmogrov-Smirnov test revealed that data had normal distribution, a t-independent test was used for statistical comparisons of the treatment groups under study (SPSS, 2020).
Results
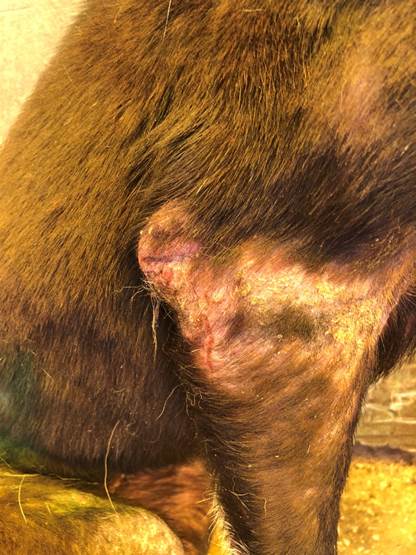
Clinical observations revealed lesions of erythematous skin with irregular alopecic areas along with scab formation. Skin lesions also had crusting and dermatitis, which appeared in various body areas of the Sarcoptes group (Figure 1). In addition, all dogs in the sarcoptic mange group had intense pruritus. We also observed that dogs in the control group had good appetite, body condition, as well as normal vital signs at clinical examination.
Microscopic examination revealed similar alterations in all diseased dogs. However, their sizes changed depending on the intensity of the Sarcoptes infection (Figure 2).
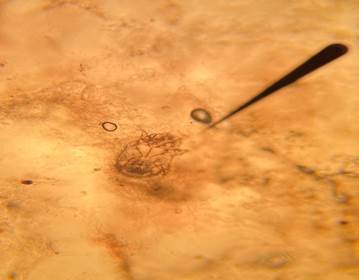
Figure 2 Sarcoptes scabiei var canis isolated from sampled dogs (10% potassium hydroxide preparation, X10 magnification).
Mean values of MDA, GSH, TAS, TOS, OSI, and NO in control and infected groups are presented in Table I. In comparison with the control group, there were significant increase in MDA, NO, OSI, and TOS levels along with significant (p<0.01) decrease in GSH and TAS levels in the Sarcoptes infested dogs.
Table 1 MDA, GSH, TAS, TOS, OSI, and NO levels in healthy and infested dogs with sarcoptic mange.
| Biochemical parameters | Groups | N | Mean | Standard error of the mean | Significance |
|---|---|---|---|---|---|
| MDA | Control | 10 | 2.0510 | 0.12084 | ** |
| Sarcoptes | 22 | 3.9268 | 0.25065 | ||
| GSH | Control | 10 | 0.4080 | 0.00998 | ** |
| Sarcoptes | 22 | 0.2214 | 0.00703 | ||
| TAS | Control | 10 | 1.8810 | 0.04418 | ** |
| Sarcoptes | 22 | 1.3145 | 0.02569 | ||
| TOS | Control | 10 | 4.4320 | 0.22611 | ** |
| Sarcoptes | 22 | 6.6536 | 0.24930 | ||
| OSI | Control | 10 | 2.3500 | 0.08539 | ** |
| Sarcoptes | 22 | 5.0773 | 0.17978 | ||
| NO | Control | 10 | 387.3000 | 7.28674 | ** |
| 22 | 464.8759 | 8.66339 |
MDA: Malondialdehyde; GSH: Glutathione; TAS: Total antioxidant status; TOS: Total oxidant status; OSI: Oxidative stress index; NO: Nitric oxide; **: p<0.01.
Hematological findings in all dogs are presented in Table 2. Significant increase (p<0.01) in WBC count in dogs of the Sarcoptes group compared to the control group was observed. On the other hand, significant decrease (p<0.01) in RBC, HGB, and PCV counts in Sarcoptes-infested dogs were found compared with the control group.
Table 2 Hematological findings in control and dogs infested with sarcoptic mange.
| Hematological parameters | Groups | N | Mean | Standard error of the mean | Significance |
|---|---|---|---|---|---|
| WBC | Control | 10 | 10.253 | 0.58423 | ** |
| Sarcoptes | 22 | 13.419 | 0.75181 | ||
| RBC | Control | 10 | 6.6 | 0.23224 | ** |
| Sarcoptes | 22 | 5.5225 | 0.16845 | ||
| HGB | Control | 10 | 14.91 | 0.57144 | ** |
| Sarcoptes | 22 | 11.945 | 0.34607 | ||
| PCV | Control | 10 | 44.514 | 1.43278 | ** |
| Sarcoptes | 22 | 32.8305 | .95314 |
WBC: White blood cell; RBC: Red blood cell; HGB: Hemoglobin; PCV: Pocket cell volume; **: p<0.01.
Discussion
Sarcoptic mange is the contagious skin disease in dogs caused by S. scabiei and is characterized by intense pruritus, alopecia, vesiculo-papular eruption, pinpoints crusts, and may cause death if left untreated (Kemp et al., 2002). In the present study, erythematous skin with irregular alopecia areas with crusting, and crusting and dermatitis on various body parts was observed. In addition, severe itching was present in all dogs in the sarcoptic mange group. The tunneling activity in the skin caused by parasites in scabies is seen as an important cause of oxidative stress (Mark et al., 2015). This study evaluated oxidative stress and changes in hematological parameters in dogs naturally infested with S. scabiei.
Free radicals are uninterruptedly created in the organism during normal metabolism. However, the production rates of free radicals increase even more in certain inflammatory disorders or syndromes. It is known that oxidative stress plays a significant role in the etiopathogenesis of various parasitic diseases in humans and animals (Chandramathi et al., 2009). Increased lipid peroxide is responsible for the pathology of skin lesions caused by Sarcoptes mites. Lipid peroxidation is an important cellular damage mechanism and is used as an indicator of oxidative stress in cells and tissues. Lipid hydroperoxides are by-products of lipid peroxidation and increased levels of lipid peroxidation products are closely associated with parasitic invasion (Kaya et al., 2007; CAM et al., 2008).
Malondialdehyde is the final compound of lipid peroxidation and is considered a key marker of oxidative stress. Increased MDA levels in peripheral blood showed exhaustion of enzymes and interrupted oxidative damage to the tissues of dogs with clinical sarcoptic mange. Parallel observations in mange cases were also described in other animal species, such as goats (De and Dey, 2010), pigs (Dimri et al., 2014), and camels (Espinosa et al., 2017).
Remarkably decreased levels of GSH, TAS, increased TOS and OSI level may be the result of overproduction of free radicals by the inflammatory cells recruited to combat the parasites, finally disrupting the antioxidant system of infested dogs. Similarly, Kocyigit et al. (2005) showed that intra and extra cellular parasites may induce or activate several oxidant-generating enzymes. Finally, inflammatory cell activation can occur in the organism. The increase in oxidant and decrease in antioxidants cannot be prevented in various diseases, and it has been reported that the oxidative/antioxidative balance shifts towards the oxidative state (Erel, 2005; Kocyigit et al., 2005; Cemek et al., 2006).
Increased NO production was also reported in humans with cutaneous leishmaniasis and other inflammatory skin diseases (Bickers and Athar, 2006). Free radicals are produced continuously by normal metabolic processes, but production rate increases during certain inflammatory or other disease conditions (Dimri et al., 2008). Once parasites are phagocytosed by macrophages, these cells produce ROS, such as O2− , H2O2, OH, and NO as a host defense mechanism (Serarslan et al., 2005). The NO increase may have been due to this mechanism.
As shown in Table 2, there were significant changes in various hematological parameters in the dogs affected by Sarcoptes mange. The dramatic decrease in circulating erythrocyte counts as well as hemoglobin amounts suggests an anemic condition. This result is quite like findings in camels (Parmar et al., 2005), goats (De and Dey, 2010), and sheep with mange (Aatish et al., 2007). It has been reported that anemia due to loss of skin proteins and leukocytosis might be due to an allergic reaction caused by mites or inflammatory reaction products (Pérez et al., 2015). The cause of anemia in the Sarcoptes group may be explained by loss of skin proteins and leukocytosis, as Pérez et al. (2015) stated.
The presence of S. scabiei on the host can cause itching and abrasions of the skin. This erosion can lead to invasion of bacteria and harmful microorganisms often causing an immune response characterized by leukocytosis (Stevanović et al., 2020). This seems to be the best explanation for the leukocytosis seen in this study.