Introduction
Water collected in rivers and springs contain great diversity of microorganisms, particulate material and dissolved organic matter. The water treatment is performed in water treatment plants (WTP). Among the types of water treatment, coagulationflocculation is the most conventional physicochemical method and widely used to remove water impurities such as suspend or colloidal particles 1. In the coagulation process occurs the rapid dispersion of chemical coagulants, generally iron or aluminum salts, which causes the destabilization of the colloids and suspended solids by rapid stirring 2. The flocculation step is necessary when the coagulation does not show total efficient in the formation of agglomerates due low-density particles. Generally, smaller particles will settle more slowly than larger particles of similar density 3.
Recently, there has been interest in natural coagulants for water treatment in developing countries. Natural coagulants are not only water clarifying agents, but they also have antimicrobial and heavy metal removal properties in some instances 4. The study of the use of natural polymers as flocculation aids has gained more prominence because of the disadvantages of synthetic polymers such as: high cost, possibility of causing environmental damage, and problems related to human health 5), (6. On the other hand, natural flocculants or bio-flocculants are non-toxic, biodegradable, stable shear, easily accessible and have low cost 7.
Okra is also known as Abelmoschus esculentus L. (Moench) or Hibiscus esculentus (L.), it is an economically important vegetable crop grown in tropical and subtropical parts of the world 8. But okra is perishable and about 30% of Brazilian production is lost and consumers identify the loss of fruit quality by the external yellowing and by the toughening or lack of tissue rupture of the apical pod when twisted with the fingers 9. However, these discarded fruits are natural flocculants for water treatment and being able to contribute to farmer income 10.
Okra mucilage is a source of natural anionic polysaccharide which is water-soluble and capable of destabilizes colloidal particles and flocculate small particles 11. The use of okra as bio-flocculant together with coagulant has been used because of its ability to increase the flotation efficiency and decantation of flocs, besides reducing the amount of coagulant 12. An increased in floc size and shorter period to achieve maximum floc size were observed when extracts of okra was used as coagulant aid, probably due to the high particle attraction capability of the extract, which in real terms could result in lower cost of water treatment 13.
Okra has shown promising results with respect to treatment of industrial textile wastewater 2. At least 97% of turbidity removal, 85% of COD (chemical oxygen demand) reduction and 93% of colour removal have been reported in these studies. Okra mucilage was effective in low concentrations and comparable to synthetic flocculants in terms of treatment efficiency 14.
In this context, this study evaluated two methods of okra preparation and the amount necessary to use them as bio-flocculant in water treatment.
Material and methods
Preparation of Okra
Okra used in this work was bought at local markets in the Southeast of Minas Gerais, in Brazil. For the extraction of mucilage, okra was cleaned, cut into small pieces and excess fiber and seeds were removed. In 12.5 g of okra was added 500 ml of distilled water and put on shaker at 200 rpm for 2 h where the mucilage was extracted. The solution was filtered for separation of fibers and viscous mucilage was collected and used as flocculant.
In the preparation powder, okra was cleaned, cut into strips and dried at 140 °C for 6 h. Okra dry was triturated in a mixer and sieved. Okra powder was selected with particle size less than 0.500 mm and solution was prepared using 12.5 g of okra powder and 500 ml of distilled water using shaker at 200 rpm for 30 min. This solution used as flocculant.
Water used in the experiments
The water used in the experiments was collected from Ribeirão Arrudas River in São Geraldo neighborhood, Belo Horizonte - MG. This river receives industry wastewater and domestic sewage of 3 cities: Belo Horizonte, Contagem and Sabará. Thus, its water has dark color, unpleasant odor and high turbidity (90.3 NTU).
Experiments in jar-test
The experiments were conducted in a jar test apparatus with 2 L of wastewater previously agitated. In the coagulation/flocculation processes were used ferrous sulfate (FeSO4) as coagulant, 40 g l-1 stock solution, and 25 g l-1 of solution of mucilage extracted or powder of okra as bio-flocculant dissolved in distillated water. The pH was adjusted to 10 adding 1.2 g of calcium hydroxide in each jar. This adjustment was necessary since ferrous sulfate (FeSO4) has higher coagulant activity in the pH range 9-11 15. Coagulant solution was added at the beginning of the tests.
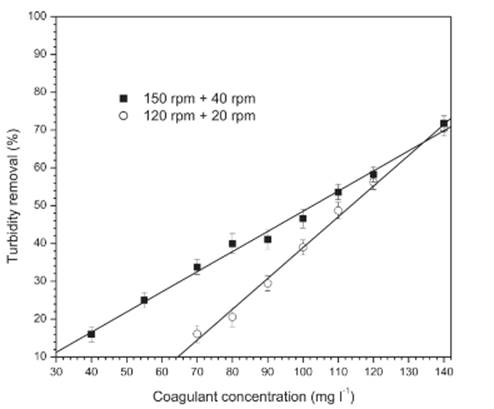
Experiments were performed with coagulant alone (without okra) and two sets of agitations were tested: 120 rpm for 1 min (fast mixing) followed by 20 rpm for 15 min (slow mixing) and 150 rpm for 1 min followed by 40 rpm for 15 min. After 30 min of stabilization, an aliquot was collected. The agitations set that shown higher turbidity removal was used in the trials with bio-flocculant.
The effect of various bio-flocculant dosages (19-87 mg l-1) was evaluation with coagulant concentrations of 40, 80 and 110 mg l-1. For each concentrations of ferrous sulfate, experiments were carried out using solutions of mucilage extracted and powder of okra. The okra solution was added at beginning of slow mixing.
Analytical measured
Turbidity was measured at beginning and stop of each experiment using a turbidimeter (HI 98703 - Hanna instruments). Samples were withdrawn using pipette from 2 cm depth and analyzed for turbidity. The percentage of turbidity removal was calculated using Equation 1.
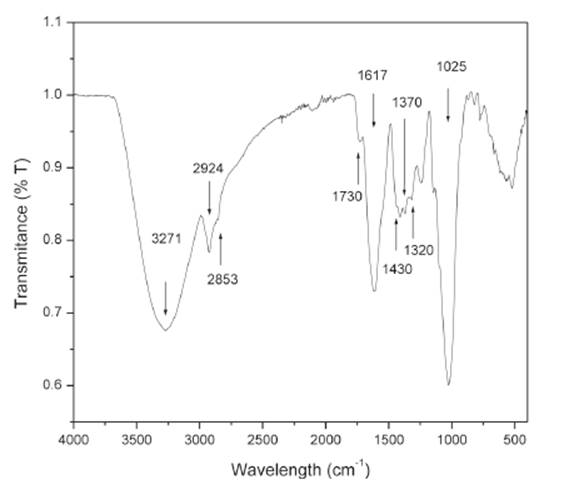
An FTIR spectrum of okra powder was obtained using an infrared spectrometer (IR Prestige-21) in the region between 4000-400 cm -1 with 4 cm resolution. Okra was pulverized before pressing into potassium bromide pellets. -1
Results and discussion
Figure 1 show turbidity removal with ferrous sulfate alone (without okra) in two sets of agitations. In both agitations sets, it is observed that increasing coagulant concentration results in higher percentages of turbidity removal. However, turbidity removal was more efficient with the agitations set 150-40 rpm. At low coagulant concentrations more intense agitation (150 rpm) is required to cause more efficient spread of coagulant which will get closer to a greater number of impurities and, consequently, form more clots that will turn into flakes and increase turbidity removal. But reaching the concentration of 140 mg l-1 of coagulant the mixing sets resulted in similar turbidity reductions around 70%. This fact occurs due to the high concentration of coagulant in wastewater.
Figure 2 shows the effect of bio-flocculant concentration using mucilage extracted and powder of okra, on turbidity removal using a fixed dosage of ferrous sulfate of 110 mg l-1, constant pH of 10 and agitations set 150-40 rpm. Note that turbidity removal using okra was greater than 80% while that ferrous sulfate alone removed 54% (for same coagulant dosage and agitation set, Figure 1). This proves that adding okra in water treatment significantly increases the turbidity removal efficiency. Note that the turbidity reduction increased gradually with the increase of bio-flocculant concentration in water until the close values were obtained. Low bio-flocculant dosage (<10 mg L-1) may not have sufficient charges for effective turbidity removal. On the other hand, high bio-flocculant dosage (>100 mg L-1), restabilization may occur due to particulate charge reversal 2. However, okra powder showed greater turbidity reduction than mucilage extracted, mainly at low okra concentrations. Okra powder has fibers together with mucilage and its small particles disperses can contribution for aggregation of molecules in water treatment and, consequently, higher effective turbidity removal. Furthermore, okra powder preparation is more agile, it allows reducing the number of preparation steps and also costs.

Figure 2 Effect of different concentrations of mucilage extracted and powder okra on turbidity reduction using dosage of ferrous sulfate of 110 mg l-1, pH=10 and agitations set 150-40 rpm.
Figure 3 shows the effect of bio-flocculant concentration, mucilage extracted and powder of okra, on turbidity removal using a fixed dosage of ferrous sulfate of 80 mg l-1, constant pH of 10 and agitations set 150-40 rpm. Note that okra powder showed greater turbidity reduction than mucilage extracted at different okra concentrations. Turbidity reductions were greater in higher bio-flocculant concentrations being that the greatest turbidity removals were 82% and 73% for powder and mucilage extracted of okra, respectively.
Reduction in the dosage of inorganic coagulants is desirable for the environment. Coagulant (ferrous sulfate) reduction of 27 % (110 to 80 mg l-1) showed small turbidity reduction (87 to 82 %) using 62 mg l-1 of okra powder. This result shows that is possible using low coagulant concentration achieve close turbidity removal. Solid precipitated with low concentration of chemical compound and higher content of organic material is more environmental friendly.
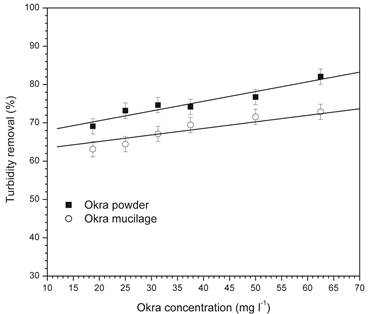
Figure 3 Effect of the concentration of mucilage extracted and powder okra on turbidity reduction using 80 mg l-1, pH=10 and agitations set 150-40 rpm.
Figure 4 shows the effect of the bio-flocculant and coagulant concentrations on turbidity removal efficiency. The turbidity removal was less than 60% at low coagulant concentrations (40 mg l-1) and increased significantly with increasing dosage of okra power. The amount of coagulant and okra powder may not have sufficient charges for particles removal. Note that substantial turbidity reduction occurs when using greater dosage of coagulant and okra powder. It is possible that using high dosage of coagulant (110 mg l-1) and bio-flocculant have excess of charges for particles removal and, consequently, less pronounced increases of turbidity removal is observed. However, optimum dosage of coagulant should be found near of 80 mg l-1. This value is much close of inorganic coagulant dosage (88 mg l-1) for maximum efficiency of COD removal, turbidity removal and color removal, found in literature 2.
It is noticed that using okra powder (62 mg l-1) allowed coagulant reduction of 110 to 80 mg l-1 keeping the turbidity reduction values higher than 80%. Using only 80 mg l-1 of coagulant was obtained only 39% of turbidity reduction while adding bio-flocculant was up to 88 %. Therefore, the combination of coagulant and bio-polymer is able to reduce the amount of chemical agent and increase the turbidity removal in the water treatment process.
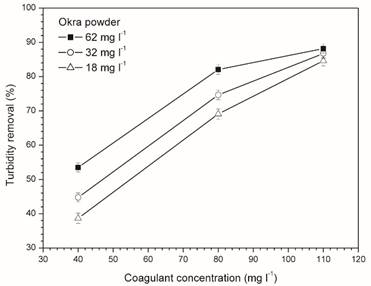
Figure 4 Effect of coagulant concentration on turbidityreduction using 18, 32 and 62 mg l-1 of okra powder,pH=10 and agitations set 150-40 rpm.
The use of okra powder favored a greater removal of suspended solids in water treatment. The process of turbidity removal is explained by electrostatic attraction (charge-charge) of okra powder and suspended particles through hydrogen bonds, van der Waals interactions or their combination 16. Okra powder is composed of organic substances formed by functional groups such as polyphenol and carboxyl that serves as actives sites for particle removal. The identification of functional groups present in okra powder was performed by infrared spectroscopy. Fig. 5 shows FTIR spectrum of okra powder in the resolution range of 4000-400 cm-1. In Figure 5, the larger band in the region of 3600-3100 cm-1 with a sharp peak centered at 3271 cm-1 is characteristic O-H stretching vibration and hydrogen bond of the hydroxyl groups 17. These groups serve as active sites for the attachment of colloidal particles and okra powder 2. Absorption peaks at 2924 and 2853 cm-1 correspond to C-H stretching vibration from methyl and methylene group in cellulose and hemicellulose components; while at 1730 cm-1 refers to the carbonyl C=O stretching vibration of unconjugated ketone, carboxylic acid and ester in lignin and hemicellulose 18. The shoulder at 1617 cm-1 may be due to the presence of water in the fibres. A little peak at 1430 cm-1 is associated to the angular deformation on the OH bonding plane. The two peaks observed at 1370 cm-1 and 1320 cm-1 in the spectrum indicate the bending vibration of C-H and C-O groups of the aromatic ring in polysaccharides. Furthermore the absorption peaks around 1200-950 cm-1 belong to polysaccharide in cellulose and indicate the presence of C-O bonds associated with the presence of functional groups such as alcohol, ether and ester 19.
Conclusion
In water treatment, the turbidity removal was more efficient with the agitations set 150-40 rpm due to best dispersion of the coagulant, especially at low concentrations. The results showed that using 80 mg l-1 of an inorganic coagulant (FeSO4) alone was removed only 39% of turbidity while adding mucilage and powder of okra went up to 76 % and 88 %, respectively. These results demonstrate the potential environmental-friendly of bio-polymer present in okra to reduce turbidity and allowed the reduction of 64 % of inorganic coagulant amount in the coagulation-flocculation process. However, the turbidity removal was more efficient when using the okra powder and it increased gradually with the increase of okra concentration in water. In okra powder were identified functional groups that serve as active sites for colloidal particles removal. Furthermore, okra powder can be prepared by simple methodology and it is great interest due to the low-cost, biodegradability and low toxicity.