Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804On-line version ISSN 2357-3791
Rev.Colomb.Quim. vol.40 no.2 Bogotá May/Aug. 2011
Electrochemical studies of certain 1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-ones
Estudio electroquímico de algunos 1-(toluenil sulfonilo)-3-amino-4-(4'-hidrazina fenil sustituidas)-2-pirazolina-5-onas
Estudo Eletroquímico de alguns 1-(toluenil sulfonila)-3-amina-4-(4'- hidrazina fenil substituídas)-2-pirazolina-5-onas
Ramana Kumar Kakarla1, Raghavendra Guru Prasad Aluru2,5, Srilalitha Vinnakota3, Narayana Swamy Golla4, Ravindranath Lakshmana Rao Krishna Rao4
1 Malla Reddy College of Engineering, Hyderabad, A.P., India,
2 ICFAI Foundation for Higher Education, Hyderabad, A.P., India
3 C.M.R. Institute of Technology, Hyderabad, A.P., India,
4 Sri Krishnadevaraya University, Anantapur, A.P., India.
Recibido: 29/07/11- Aceptado: 26/08/11
Abstract
The electrochemical behavior of certain 1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-ones were studied at the dropping mercury electrode by employing DC polarography. The variables that influence the electrode process were extensively studied. All compounds under investigation gave two well-defined polarographic waves. The mechanism for the electrode process was proposed in acid as well as in basic media.
Key words: Polarographic studies, 1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-ones, influence of variables, mechanism of electrode reaction.
RESUMEN
El comportamiento electroquímico de ciertos 1-(Toluenil sulfonil)3-amino-4-(aril hidrazonio)-2-pirazolin-5-onas sustituidas fueron estudiados con un electrodo de gota de mercurio empleando polarografía de corriente directa. Las variables que influyen sobre el proceso en el electrodo fueron estudiadas ampliamente. Todos los compuestos estudiados presentaron dos ondas polarográficas bien definidas. Se propone un mecanismo para las reacciones electroquímicas estudiadas tanto en medio ácido como básico.
Palabras clave: Estudios polarográficos, 1-(Toluenil sulfonil)3-amino-4-(aril hidrazonio sustituidos)-2-pirazolin-5-onas, influencia de variables, mecanismos de reacción en el electrodo.
RESUMO
O comportamento eletroquímico de certos 1-(Toluenil sulfonil)3-amino-4-(aril hidrazonio)-2-pirazolin-5-onas substituídas foram estudados com um eletrodo de gota de mercúrio empregando polarografia de corrente direta. As variáveis que influem sobre o processo no eletrodo foram estudadas amplamente. Todos os compostos estudados apresentaram duas ondas polarograficas bem definidas. Propõe-se um mecanismo para as reações eletroquímicas estudadas tanto em meio ácido quanto em meio básico.
Palavras chave: Estudos polarograficos, 1-(Toluenil sulfonil)3-amino-4-(aril hidrazonio substituídos)-2-pirazolin-5-onas, influência de variáveis, mecanismos de reação no eletrodo.
INTRODUCTION
Pyrazole and its derivatives are important class of heterocyclic compounds due to their wide spread applications in medicinal (1) and pesticide chemistry (2, 3). Compounds containing pyrazole ring system are known to display diverse pharmacological activities such as antibacterial (4), antifungal (2), antiviral (5), antiinflammatory (6, 7), analgesic (6, 7), and antipyretic (6). Some of them have been used as antihyperglycemic agents (8) or cardiovascular drugs (9), while some of them have been used as anxiolytic drugs (10). A systematic study of this class of compounds reveled that their pharmaceutical activity results from the presence of pyrazole nucleus. Moreover, the biological activities of pyrazol-5-ones depend on the nature of the substituents. Various therapeutic aspects and numerous applications of above said class of compounds have inspired the authors to understand their reduction process at the dropping mercury electrode. However, the knowledge of reduction is very important to understand their biological activity.
Pyrazolin-5-ones are also used in dye industry (11) and as indicators (12) in complexometric titrations. Many pyrazoles have been found to function as luminescent and fluorescent (13, 14) agents.
EXPERIMENTAL
All chemicals and solvents used were of analytical reagent grade procured from Merck, India. Double distilled water was used for the preparation of solutions. Working solutions were prepared by appropriate dilutions of the standard solution.
DC recording polarograph manufactured by ELICO Private Limited, Hyderabad, India was used for polarographic studies. The current voltage measurements were performed with three-electrode assembly, a dropping mercury electrode as working electrode, calomel as reference electrode and platinum electrode as counter electrode. The current responses and applied potentials were recorded at scan rate 100 mV/min. The dropping mercury electrode had the capillary characteristics, m = 2.422 mg/s, t = 2.5 s, h = 60 cm. pH measurements were made using pH meter Model L1-10 manufactured by ELICO Private Limited, Hyderabad, India.
Synthesis pyrazolin-5-ones
A mixture of appropriate diazonium cyano ester and toluene sulfonyl hydrazide in ethanol was refluxed for six hours and cooled. The crystalline solid separated was filtered, washed with water, dried and recrystallysed from dimethylformamide (1:1). The physical characteristics of the different compounds synthesized are presented in Table 1.
General experimental procedure
10 mL of buffer solution of required pH, 2.5 mL of pyrazolin-5-one (1x10-2 M) and 10 mL of dimethylformamide were taken into the polarographic cell. The solution was made to a total volume of 25 mL with distilled water. Polarograms were recorded after deaeration with nitrogen gas.
RESULTS AND DISCUSSION
1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-ones (A-D) exhibit two waves in the pH range 1.1-10.1. A decrease in wave height with increase in pH was observed for all the compounds. Among the three sites susceptible for reduction namely the exocyclic >C=N, cyclic >C=N and cyclic amide, exocyclic >C=N is more susceptible for reduction than cyclic >C=N and cyclic amide as it was experimentally confirmed that 1-(Toluenyl sulfonyl)-3-amino- 2-pyrzolin-5-one does not undergo reduction under the experimental conditions. Hence the polarographic reduction of 1-(Toluenyl sulfonyl)-3-amino-4-(substituted aryl hydrazono)-2-pyrazolin-5-ones is due to the reduction of exocyclic >C=N group.
Half-wave potential- pH relation
The compounds (A-D) exhibit two well-defined waves in the entire pH range 1.1-10.1 of study. The half-wave potentials of the first and second waves for all the compounds turn more negative with increase in pH in the pH range 1.1-7.1 and remain constant in alkaline medium (8.1-10.1).
The E1/2-pH plots are shown in Figure 1(I), (II) and (III). The E1/2-pH relationship for the first wave is represented by
a) -E1/2 = 0.05 + 0.08172 x pH V vs SCE
b) -E1/2 = 0.03 + 0.08145 x pH V vs SCE
c) -E1/2 = 0.06 + 0.08372 x pH V vs SCE
d) -E1/2 = 0.08 + 0.08546 x pH V vs SCE
The E1/2-pH relationship for the second wave is represented by
a) -E1/2 = 0.21 + 0.08172 x pH V vs SCE
b) -E1/2 = 0.15 + 0.08956 x pH V vs SCE
c) -E1/2 = 0.21 + 0.08372 x pH V vs SCE
d) -E1/2 = 0.24 + 0.08567 x pH V vs SCE
The fact that the half-wave potential values did not vary with pH in alkaline medium suggests that the protons were involved in the reduction process. The number of protons (P) involved in the reduction process was calculated using the equation
where a is transfer coefficient and na is number of electrons involved. The fractional value of P presented in Table 2 at different pH values suggests the heterogeneous proton transfer in the reduction process (15).
Effect of mercury column height (h) on the wave height (H)
The wave height (H) linearly varies with h1/2 and the plot of h1/2 vs H passes through the origin. This suggests the diffusion controlled nature of the wave.
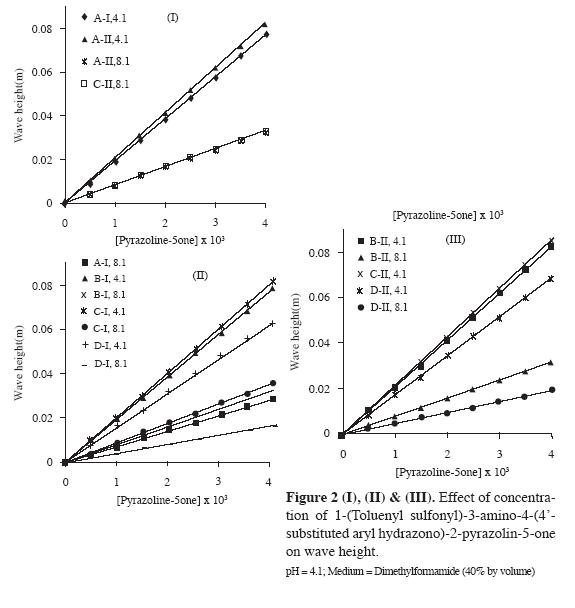
Effect of concentration (C) of the depolariser on the wave height (H)
The effect of concentration of pyrazolin-5-ones (A-D) on the wave height in the range 0.5-4.0 mM in the typical pH media 4.1 and 8.1 was studied. The linear wave height-concentration plots (Figure 2 I, II and III) passing through the origin revealed that the reduction process was diffusion controlled and the method can be applied to determine the trace amounts of pyrazolin-5-ones under study.
Irreversible nature of the electrode process
The irreversible nature of the waves may be attributed to the bulky aryl hydrazono group at the end of >C=N linkage (16). Semi log plots shown in Figure 3 (I) and (II) established the irreversible nature of the wave. The fractional value of the slope 0.049-0.051 and 0.048-0.053 respectively for first and second waves suggests that the reduction process was irreversible in nature. The irreversible nature of the waves was further supported by the shift of the half-wave potential values towards the more negative values with increase in the concentration of the depolarizer (17), the decrease in heterogeneous rate constant values Kand an increase in increase in activation free energy change values ΔG° with increase in the pH of the solution (Table 2).
Effect of pH on wave height
The effect of pH on wave height is presented in Figure 4 (I) and (II). The plots for the first and second waves for all the compounds (A-D) assumed the shape of dissociation curve. This type of behavior is expected if the depolarizer undergoes chemical cleavage in the acidic or alkaline medium.
Kinetic parameters of electrode reaction
The heterogeneous rate constant, Kf,h and activation free energy change, ΔG° in different pH media (1.1-10.1) for the compounds under study are presented in Table 2. As reveled from the Table 2, the decrease in Kf,hand the increase in ΔG° value with increase in pH are indicative of the enhanced irreversible nature of the reduction process with the increase in pH.
Effect of temperature
The polarograms of pyrazolin-5-ones in a media of pH 4.1 were recorded at 303K, 313K, 323K and 333K, to study the effect of temperature on the half-wave potential and wave height. The results are presented in Table 3. The compounds under study exhibit two well-defined waves at all temperatures studied (303-333K) at pH 4.1. The wave height increases with increase in temperature. The temperature coefficient values were in the range 0.734-1.495% deg-1 and were in good agreement with the values reported in the literature (18) for other similar compounds.
The table reveals that the anavalues decrease as the temperature increases from 303K to 333K. The decrease in ana values with increase in temperature was due to decrease in a value. The decrease in a values indicates that the transfer of electrons was made increasingly difficult with the increase in temperature. Hence, the system tends to become more irreversible (19) with the increase in temperature. This fact was further supported by the shift of half-wave potentials towards more negative values with raise in temperature. Literature survey (20) reveals the similar observations for similar compounds.
The formal rate constant (Kf,h) calculated at different temperatures is shown in Table 4. Stoke-Einstein equation was used to calculate diffusion coefficient necessary for the calculation of the formal rate constant at different temperatures. Table 4 shows that the formal rate constant decreases with increase in temperature. The decrease in K with increase in temperature suggests that the electrode reaction was becoming increasingly irreversible with the raise in temperature. This observation was in accordance with the conclusion arrived on the basis of ana values.
Millicoulometry
The number of electrons involved in the reduction of pyrazolin-5-ones was evaluated in Britton-Robinson buffers containing 40% (v/v) dimethylformamide. The millicoulometer of De Vries and Kroon (21) with mercury pool cathode was employed to determine the value of n'. The results are presented in Table 5.
Reduction mechanism
An inspection of the results presented in Table 2 reveal that the compounds A-D exhibit two 2-electron reductive waves in the pH range 1.1-10.1. It was evident from the polarographic reduction of semicarbazones and hydrazones (22) that N-N bond in hydrazono group (>C=N-NH-) was reduced more easily than the azomethine (>C=N-) group.
In acidic medium, the first step involves the two-electron reductive cleavage of N-N bond leading to the formation of 1-(Toluenyl sulfonyl)-3-amino-4-imino-2-pyrazolin-5-ones (23, 24) (II) and substituted aniline. The second step involves two-electron reduction of ketimine (II) to the corresponding diamine (III). The wave height of both these steps was affected by acid-base equilibrium. The variation of wave height with pH was similar to the trend reported in the literature (25). Therefore, reduction mechanism shown in Figure 5 (I) was proposed in acidic medium.
In alkaline medium, the azomethine group (>C=N-NH-) of the compounds exist in the azomethine anionic form (>C=N-) (26). The first wave was attributed to the two-electron reductive cleavage of N-N bond in azomethine anionic form (IV) which was susceptible for cleavage to the corresponding heterocyclic carbonyl compound. The second wave was due to the two-electron reduction of heterocyclic carbonyl compound to the corresponding alcohol. Therefore, reduction mechanism shown in Figure 5(II) was proposed in alkaline medium.
Effect of substituents on the polarographic reduction
Heyrovsky (27) was the first man to correlate the polarographic behaviour of a representative number of compounds with their structure. Structural correlations are usually done with sp values for the compounds in an aromatic series. E1/2 - sp plots for the compounds under investigation are presented in Figure 6 (I) and (II). The values of specific reaction constant (r) calculated from the slopes of E1/2 - sp plots are presented in Table 6.
The discussion of the effect of substituents in terms of the Hammett equation was possible because ΔE1/2/ΔpH, ‹na and I (diffusion current constant) values (Table 2) were practically in the same range for the entire reaction series. The values of the Hammett substituent constant were taken from the literature (28). The values of r were found to be in the range of 0.10-0.30. Positive and low values (29) of r indicate that the polarographic reduction involves a nucleophilic addition of electron to the substrate. This fact confirms that the electron uptake process was the potential rate determining step in all the reduction processes studied.
Effect of cation on the polarographic reduction of 1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-ones
Not much work is reported (30) on the effect of cation on r values of the polarographic reduction. Few studies were reported on the reduction of benzylidine acetone (31) and nitrobenzene (32). The r values presented in Table 7 increases with increase in the size of cation. This implies that the susceptibility for nucleophilic addition diminishes with increase in the size of the cation. Similar results were reported for benzylidine acetones (31) and N'-Benzyl sulfonyl arylazo pyrazoles (33).
Effect of organic co-solvent on the polarographic behaviour of 1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-ones
The polarograms of the 1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-ones were recorded at pH 4.1 in 50 and 75% v/v aqueous solutions of dimethylformamide (DMF), dimethylsulfoxide (DMSO), acetonitrile (CH3CN) and methyl alcohol (CH3OH). The results are presented in Table 8. These results indicate that the change in composition of the solvent does not bring any change in the number of waves and the shape of the polarograms. However, a change in the position of the wave on the potential axis and a marked decrease in the diffusion current was observed. The decrease in the diffusion current may be attributed to the decrease in the effective diffusion coefficient value. This may be due to the increase in the viscosity of the solution or the change in the size of the solvated species (34). In the presence of organic solvents, the half-wave potential values were shifted to values that are more negative and the magnitude of the shift depends on the nature of the solvent. The shift was in the order, DMSO < DMF < CH3CN < CH3OH, and parallels the trend in dielectric constant of solvents. The observed shift in E1/2 can be attributed to several factors such as dielectric constant, solvation of ions and adsorption of organic solvent on the electrode surface.
The diffusion-controlled nature of the polarographic wave in the presence of organic solvent was evident from the linear plot of H versus h1/2 passing through the origin. The semi log plots (-Edme Vs log i / id - i) were linear and their slopes were more than the theoretically expected values for reversible waves. This indicates the irreversible nature of the electrode reaction in the presence of organic solvent. This shows that the mechanism of the electrochemical reaction is similar even in the presence of organic solvents although a marked shift in the diffusion current and the half-wave potentials were observed.
Effect of surfactants on the polarographic behaviour of 1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-one
Pyrazoles have immense medicinal and biological importance and hence knowledge of the effect of surfactants on their redox behaviour at the solution mercury interface may prove very useful from the physiological point of view (35). The effect of surfactants on redox behaviour of these compounds is of immense importance since the surfactants are used as emulsifiers in drugs. Malik and Rajeev Jain 1982 (36) reported that the addition of surfactants beyond the concentration just sufficient to eliminate the maximum has affected the reversibility of the electrode reaction.
So, in the present studies the effect of different surfactants on the polarographic reduction of 1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-ones has been investigated in solutions of pH 4.1. The results show that the reduction becomes increasingly difficult and the wave height decreases with increase in the concentration of the surfactants. The half wave potentials shift to values that are more negative and was due to the preferential adsorption of the surfactant at dropping mercury electrode. This resulted in the partial desorption of the depolariser from the electrode surface and thus lowering the surface concentration of the depolarizer (37). Similar observations were reported in the literature (38).
CONCLUSION
The reduction of 1-(Toluenyl sulfonyl)-3-amino-4-(4'-substituted aryl hydrazono)-2-pyrazolin-5-ones leads to two irreversible and diffusion-controlled polarographic waves. The kinetic parameters of the electrode process were evaluated and presented. The effect of substituents, cations, solvents and surfactants on the reduction process were detailed. Based on the results obtained, a plausible reduction mechanism was proposed.
Acronyms
The short form ‘A-I, 4.1’ indicated in the figures, stands for ‘compound-nth wave, pH’ i.e. A represents the compound, I represents first wave and 4.1 represents the pH.
REFERENCES
1. Haddad, N.; Salvango, A.; Busacca, C. Application of the Palladium-Catalyzed N-Arylation of Hydrazones to Deactivated Heteroaryl Halides in the Synthesis of Pyrazoles. Tetrahedron Lett. 2004. 45: 5935-5937. [ Links ]
2. Bosum-Dybus, A.; Neh, H. Preparation of enantiomerically pure pyrazolines by an enantioconvergent process. Ann. Chem. 1991. 8: 823-825. [ Links ]
3. Jelich, K.; Brandes, W.; Hanssler, G.; Reinecke, P. Substituted pyrazolin-5-ones, composition containing, and method of using them to combat fungi. 1988. 06/925184. [ Links ]
4. Tanitame, A.; Oyamada, Y.; Ofugi, K.; Fujimoto, M.; Iwai, N.; Hiyama, Y.; Suzuki, K.; Ito, H.; Terauchi, H.; Kawasaki, M.; Nagai, K.; Wachi, M.; Yamagishi, Jun-ichi. Synthesis and antibacterial activity of a novel series of potent DNA gyrase inhibitors. Pyrazole derivatives. J. Med. Chem. 2004. 47: 3693-3696. [ Links ]
5. Kedar, R. M.; Vidhale, N. N.; Chincholkar, M. M. Synthesis of new heterocyclics and their antimicrobial studies. Orient J Chem. 1997. 13: 143-148. [ Links ]
6. Tsurumi, K.; Abe, A.; Fujimura, H.; Asai, H.; Nagasaka, M. General pharmacological actions of l-(m-chlorophenyl)-3-N,N-dimethylcarbamoyl-5-methoxypyrazole (PZ-177), Folia Pharmacol. Jpn. 1976. 72: 41-52. [ Links ]
7. Mariappan, G.; Saha, B.P.; Suthatson, L.; Haladar, L. Synthesys and biological evaluation of pyrazolone derivatives. Indian Journal of Chemistry. 2010. 49B: 1671-1674. [ Links ]
8. Kees, K.L.; Fitzgerald, J.J.; Steiner, K.E.; Mattes, J.F.; Mihan, B.; Tosi, T.J. New potent antihyperglycemic agents in db/db mice: Synthesis and structure activity relationship studies of (4-Substituted benzyl)(trifluoromethyl)pyrazoles and-pyrazolones. Med. Chem. 1996. 39: 3920-3926. [ Links ]
9. Yukihito, H.; Daisuke, J.; Kazuaki, C.; Masao, Y. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases. Recent Patent Cardiovasc. Drug Discov. 2006. 1: 85-93. [ Links ]
10. Athina, G.; Babaev, E.; Dearden, J.; Dehaen, W.; Filimonov, D.; Galaeva, I.; Krajneva, V.; Lagunin, A.; Macaev, F.; Molodavkin, G.; Poroikov, V.; Pogrebnoi, S.; Saloutin, V.; Stepanchikova, A.; Stingaci, E.; Tkach, N.; Vlad, L.; Voronina, T. Design, synthesis, computational and biological evaluation of new anxiolytics. Bioorg. Med. Chem. 2004. 12: 6559-6568. [ Links ]
11. Metwally, M.A.; Khalifa, M.E.; Amer, f.a. New azodisperse dyes with 4-hydroxymethyl-2-pyrazolin-5-one ring for dyeing polyester fabrics. Dyes and Pigments. 2008. 76: 379-385. [ Links ]
12. Luminita, V.; Lerch-Gurguta, R.; Badea, I; Marinescu, V. Spectral behavior of an azopyrazolonic derivative and its use as a new metal indicator for Ti(IV). Acta Chim. Slov. 2002. 49: 623-629. [ Links ]
13. Yang, J.G.; Ge, H. M.; Jie, N. Q.; Ren, X. Z.; Wang, N. X.; Zou, H.B. Study on the co-luminescence system of terbium - gadolinium - 4,4'-(1,6-dioxohexamethylene)bis-(3-methyl-1-phenyl-2-pyrazolin-5-one) hexcadecyltrimethylammonium bromide and its analytical applications, Spectrochim. Acta, Part A. 1995. 51A: 185-195. [ Links ]
14. Zheng, Z.; Yu, Z.; Luo, N.; Han, X. Tautomerism-dependent ring construction of N-heterocyclic compounds from the reactions of 1-alkynyl Fischer carbene complexes and substituted pyrazolinones. J Org Chem. 2006. 71: 9695-9700. [ Links ]
15. Ravindranath, L.K.; Rama Das, S.R.; Brahmaji Rao, S. Polarographic behaviour of arylazo pyrazoles. Electochimica Acta. 1983. 28: 601-603. [ Links ]
16. Malik, W.V.; Goyal, R.N.; Jain, R. Polarographic reduction of some arylazopyrazoles in N,N'-dimethylformamide. J. Electroanal. Chem. 1978. 87: 129-135. [ Links ]
17. Meites, L. Polarographic Techniques, 2nd ed. New York: Interscience. 1967. p. 234. [ Links ]
18. Meites, L. Polarographic Techniques. 2nd ed. New York: Interscience. 1967. p. 139. [ Links ]
19. Sharma, S.S.; Singh, M. Polarographic studies on the influence of concentration and nature of different supporting electrolytes on the kinetics of irreversible electrode process of Ni(II) and Co(II). J. Indian Chem. Soc. 1979. 56: 183-191. [ Links ]
20. Ram, R.; Singh, M. Polarographic reduction of nitrobenzene at different temperatures. Indian J. Chem. 1979. 18A: 69-71. [ Links ]
21. De Vries, T.; Kroon, J.L. A millicoulometer method for the determination of polarographic n-values. J. Am. Chem. Soc. 1953. 75: 2484-2486. [ Links ]
22. Kitayev, Y.P.; Budnkov, G.K.; Nalsora, L.I. Study of the structure and reactivity of nitrogen-containing derivatives of carbonyl compounds. Izv. Akad. Nauk. SSSR, Serv. Khiw. 1967. 9: 1911-1917. [ Links ]
23. Millefiori, S.; Campo, G. Polarographic reduction of azo compounds. I. 1-phenylazo-2-naphthols. Ann. Chim., (Rome). 1969. 59: 128-137. [ Links ]
24. Millefiori, S. Polarographic reduction of azo compounds. II. 4-phenylazo-2-naphthols., Ann. Chim., (Rome). 1969. 59: 138-154. [ Links ]
25. Cordes, B.H.; Jemcks, W.P. The mechanism of hydrolysis of schiff bases derived from aliphatic amines. J. Am. Chem. Soc. 1963. 85: 2843-2848. [ Links ]
26. Prasad, A.R.G.; Seshagiri, V.; Ravindranath, L.K. Polarographic investigations of certain propiophenone benzoic acid hydrazones. Jordan Journal of Chemistry. 2011. 6: 51-64. [ Links ]
27. Heyrovsky, J. Polarography. Wien: Springer Verlag. 1941. p. 185. [ Links ]
28. Jaffe, H.H. A reexamination of the Hammett equation. Chem. Rev. 1953. 53: 191-261. [ Links ]
29. Hammett, L.B. Physical Organic Chemistry. New York: McGraw-Hill. 1940. p. 184. [ Links ]
30. Mairanovskii, S.G. Effect of the double layer structure and of the adsorption of electrode reaction participants up on polarographic waves in the reduction of organic substances. J. Electroanal. Chem. 1962. 4: 161-181. [ Links ]
31. Sato, Polarography of nitro and hydrazine compounds. H. Bull. Natl. Hyg. Lab. 1957. 75: 58-65. [ Links ]
32. Holleck, L.; Becher, D. Influence of ions of supporting electrolyte on polarographic reduction of aromatic nitro compounds in acetonitrile and DMF, J. Electroanal. Chem. 1962. 4: 321-331. [ Links ]
33. Jain, R.; Sudha, R.; Goyal, R.N. Polarographic behaviour of some potential antidiabetic benzylsulphonylarylazopyrazoles, Electrochim. Acta. 1981. 26: 1519-1523. [ Links ]
34. Patai, S. The Chemistry of the hydrazo, azo and azoxy groups. London: Interscience, Part I. 1964. p. 479. [ Links ]
35. Pillman, B.; Pullman, A. Quantum Biochemistry. New York: John Wiley and Sons. 1963. p. 202. [ Links ]
36. Malik, W.U.; Rajeev, J. Polarographic studies on effect of some ionic and nonionic surfactants on the kinetics of irreversible electrode process of arylazopyrimidine and arylazopyrazole. J. Indian Chem. Soc. 1982. LIX: 962-964. [ Links ]
37. Kastening, B.; Cartmann, H.; Holleck, L. Úber die wirkungsweise von inhibitoren bei elektrochemischer reduktion organischer verbindungen-III. Faraday-Admittanz-messungen zur ermittlung der adsorptionsverdrängung. Electrochemica Acta. 1864. 9: 741-755. [ Links ]
38. Parsons, R. The effect of specific adsorption on the rate of an electrode process. J. Electroanal. Chem. 1969. 21: 35-43. [ Links ]