Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804
Rev.Colomb.Quim. vol.42 no.2 Bogotá May/Aug. 2013
Aqueous citric acid as green reaction media for the synthesis of octahydroxanthenes
Ácido citrico acuoso como medio de reacción "verde" en la síntesis de octahidroxantenos
Ácido cítrico aquoso como meio de reação verde para a síntese de octaidroxantenos
Camilo A. Navarro D.1 Cesar A. Sierra1 and Cristian Ochoa-Puentes1*
1 Departamento de Química, Universidad Nacional de Colombia, Bogotá D.C. Colombia
Corresponding author. Tel. +57 1 3165000; fax: +57 1 3165220.
E-mail address: cochoapu@unal.edu.co (C. Ochoa-Puentes).
Recibido: mayo 7 de 2013 Aceptado: julio 10 de 2013
Abstract
A simple, convenient and environmentally friendly one-pot procedure for the synthesis of 1,8-dioxo-octahydroxanthenes by the reaction of dimedone and aromatic aldehydes in aqueous citric acid is described. In this green synthetic protocol promoted by the reaction media, the use of any other catalysts and hazardous organic solvents are avoided, making the work up procedure greener and easier. The isolation of the products, obtained in good yields, is readily performed by filtration and crystallization from ethanol when required and the aqueous acidic media can be easily recycled and reused several times without significant loss of catalytic activity.
Keywords: Citric acid; dimedone; green synthesis; octahydroxanthenes.
Resumen
En esta contribución se describe un procedimiento one-pot simple, conveniente y medioambientalmente amigable para la síntesis de 1,8-dioxo-octahidroxantenos por la reacción de dimedona y aldehídos aromáticos en ácido cítrico acuoso. En este protocolo de síntesis "verde" promovido por el medio de reacción, el uso de otros catalizadores y solventes orgánicos peligrosos es eliminado, haciendo el tratamiento final de la reacción más fácil y "verde". El aislamiento de los productos, obtenidos en buenos rendimientos, se lleva a cabo mediante filtración y recristalización en etanol cuando es necesario, y el medio acuoso ácido puede ser reciclado y reutilizado varias veces sin pérdida significativa de la actividad catalítica.
Palabras clave: Ácido cítrico; dimedona; síntesis verde; octahidroxantenos.
Resumo
É descrito um procedimento simples, conveniente, e ambientalmente amigável para a síntese de 1,8-dioxo-octaidroxantenes pela reação de dimedona e aldeídos aromáticos em ácido cítrico aquoso em uma só etapa. Em este protocolo de síntese verde, promovido pelo meio de reação, a utilização de algum outro catalisador e solvente orgânico perigoso é evitada, fazendo o procedimento mais fácil e mais "verde". O isolamento dos produtos, obtidos em grande rendimento, é feito por meio de filtração e cristalização a partir de etanol quando é requerido e o meio aquoso ácido pode ser facilmente recuperado e reutilizado várias vezes sem perder significativamente sua atividade catalítica.
Palavras-Chave: Ácido cítrico; dimedona; síntese verde; octaidroxantenos.
Introduction
Xanthenes are a very interesting class of oxygen-containing heterocycles with a large number of naturally occurring and synthetic derivatives.(1-6) A variety of xanthenes and derivatives based on this nucleus exhibit potential applications in the field of medicinal chemistry and materials science as anti-viral,(7 ) anti-bacterial,(8) and anti-inflammatory agents,(9) as fluorescent materials,(10) in photodynamic therapy,(11,12) dyes,(13) luminescent sensors,(14) and laser technologies.(15)
Recently enormous efforts has been made in expanding the scope of the synthesis of 1,8-dioxo-octahydroxanthenes, being the condensation of the appropriate aldehyde and dimedone or 1,3-ciclohexanedione the commonly performed method for obtaining this heterocyclic compound. Several synthetic procedures using alternative solvents,(16) solvent-free conditions with different catalysts,(17-23) ionic liquids,(24-27) homogeneous(28-30) and heterogeneous(31-38) catalysts, as well as microwave-(39) and ultrasound- assisted(40-44) synthesis have been reported. Although, many of the reported methods are effective, some of them require a previous preparation of the catalyst or involve the use of expensive reagents, toxic solvents, tedious work-up, low yields, long reaction times and harsh reaction conditions.
Citric acid is a low-cost, non-volatile, stable, readily available weak organic acid produced microbially by various fermentation processes, substrates and microorganisms.(45) It is a natural preservative and antioxidant available in vegetables and fruits, most notably in citrus fruits where it constitutes around 8% of the dry weight. In food chemistry, this acid has been used in the production of candies, fruit juice, ice cream, marmalade, jelly, also to supress the browning process in fruits and as a synergetic compound for antioxidants.(46) Citric acid forms a wide range of salts and complexes with metals like copper, iron, manganese, magnesium and calcium, being this the reason for its use as a sequestering agent in industrial processes and as an anticoagulant blood preservative. In fats and oils citric acid acts as antioxidant reducing the metal-catalysed oxidation by chelating traces of metals such as iron. Other pharmaceutical and industrial applications include antioxidant (vitamins and cosmetics), salt formation (iron preparations), sequestering (cleaning metals, polimerizations) and buffering (detergents and photography).(47) In the field of organic synthesis, this acid has been used as efficient promoter of the Beckmann rearrangement,(48),(49) and for the synthesis of dihydropyrimidin-(2H)-ones,(50) 2,3-dihydroquinazolin-4(1H)-ones(51) and quinolones.(52)
Recently, we described the simultaneous synthesis and photophysical characterization of decahydroacridine-1,8-diones and 1,8-dioxo-octahydroxanthenes where a competitive one-pot reaction yielding both products was achieved under refluxing acetic acid.(53) In connection with our ongoing studies on the photophysical properties of chromophoric compounds(54) and motivated for the aforementioned results, we decide to develop a green synthetic methodology, in which a selected group of xanthene derivatives could be obtained avoiding harsh conditions.
In the present work, we report an efficient, convenient and environmentally friendly one pot procedure for the synthesis of 1,8-dioxo-octahydroxanthenes by the use of aqueous citric acid as a green reaction media which promoted the reaction.
Materials and methods
General
All chemicals were purchased from Merck, Aldrich and Fluka Chemical companies and used without further purification. Absolute ethanol and glacial acetic acid were used as solvent for some reactions. Solutions of organic acids were prepared in distilled water. Melting points were determined using open glass capillaries on a Stuart SMP10 melting point apparatus and are uncorrected. The infrared spectra (IR) were recorded using a Shimadzu IR prestige 21 FT-IR instrument with potassium bromide pellets. The 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were measured in CDCl3 using a Bruker Avance 400 spectrometer and chemical shifts are expressed in δ ppm using TMS as internal standard. HPLC-MS analyses was performed in a Shimadzu LCMS-IT-TOF equipment (Shimadzu, Kyoto, Japan) equipped with an electrospray ionisation (ESI) probe. The electrospray ionization (ESI) probe was operated simultaneously in the positive and negative mode: CDL, 250°C; block at 200 °C; flow gas (N2) at 1.5 L/min; CDL voltage, 1.8 kV; ion accumulation, 20 ms; and scan range m/z, 100-2000 u. The energy of the collision gas (Argon) was fixed at 50%. LCMS Solution software was used for data collection and analysis. All reactions were conducted in a 5 mL capped flask equipped with a magnetic stirring.
General synthetic methodology
A mixture of the selected aldehyde (0.5 mmol) and 5,5-dimethylcyclohexane-1,3-dione (1.0 mmol) in 0.3 M citric acid (2.0 mL) was stirred at 90 °C for the appropriate time. After reaction completion (TLC), the formed solid was filtered, washed with water and recrystallized from ethanol when required.
Selected spectral data
3,3,6,6-Tetramethyl-9-phenyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8-(2H)-dione 3a.
White solid. Yield 85% (148 mg, 0.42 mmol), mp 204–206 oC. FT-IR (KBr pellet, cm-1): 2956, 2854, 1662, 1491. 1H NMR (400MHz, CDCl3) δ (ppm): 7.30 (dd, 2H, 3J= 7.0 Hz, 4J= 1.5 Hz), 7.23 (t, 2H, 3J= 7.2 Hz), 7.12 (t, 1H, 3J= 7.3 Hz), 4.77 (s, 1H), 2.48 (s, 4H), 2.25 (d, 2H, 2J= 16.3 Hz), 2.18 (d, 2H, 2J= 16.3 Hz), 1.12 (s, 6H), 1.01 (s, 6H). 13C NMR (100 MHz, CDCl3) δ (ppm): 196.3, 162.2, 144.1, 128.3, 128.0, 126.3, 115.6, 50.7, 40.8, 32.2, 31.8, 29.2, 27.3. HRMS (ESI-TOF) calcd. for C23H27O3 : [M+H]+: 351.1882; found: 351.1882.
9-(4-(Dimethylamino)phenyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione 3c.
White solid. Yield 80% (157.4 mg, 0.40 mmol), mp 219-221 oC. FT-IR (KBr pellet, cm-1): 2952, 2875, 1659, 1572. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.14 (d, 2H, 3J= 8.8 Hz), 6.60 (d, 2H, 3J= 8.7 Hz), 4.66 (s, 1H), 2.87 (s, 6H), 2.45 (s, 4H), 2.23 (d, 2H, 2J= 16.3 Hz), 2.17 (d, 2H 2J= 16.3 Hz), 1.09 (s, 6H), 1.00 (s, 6H). 13C NMR (100 MHz, CDCl3) δ (ppm): 196.6, 161.8, 128.9, 116.0, 112.4, 50.7, 40.8, 40.7, 32.2, 30.6, 29.2, 27.4. HRMS (ESI-TOF) calcd. for C25H32NO3 : [M+H]+: 394.2304; found: 394.2333.
9-(4-Chlorophenyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione 3g.
White solid. Yield 83% (159.5 mg, 0.41 mmol), mp 230-232 oC. FT-IR (KBr pellet, cm-1): 2954, 2875, 1661, 1573. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.13-7.24 (m, 4H), 4.71 (s, 1H), 2.46 (s, 4H), 2.21 (m, 4H), 1.10 (s, 6H), 0.99 (s, 6H). 13C NMR (100 MHz, CDCl3) δ (ppm): 196.3, 162.4, 142.7, 132.0, 129.8, 128.2, 115.83, 50.7, 40.9, 32.2, 31.5, 29.3, 27.3. HRMS (ESI-TOF) calcd. for C23H26ClO3 : [M+H]+: 385.1492; found: 385.1501.
3,3,6,6-Tetramethyl-9-(2-nitrophenyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione 3i.
Yellow solid. Yield 78% (154.2 mg, 0.39 mmol), mp 258-261 oC. FT-IR (KBr pellet, cm-1): 2954, 2935, 1662, 1515, 1463. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.76 (d, 1H, 3J= 7.3 Hz), 7.43 (m, 1H), 7.37 (d, 1H, 3J= 7.4 Hz), 7.24 (m, 1H), 5.51 (s, 1H), 2.46 (s, 4H), 2.23 (d, 2H, 2J= 16.3 Hz), 2.16 (d, 2H, 2J= 16.3 Hz), 1.10 (s, 6H), 1.01 (s, 6H). 13C NMR (100 MHz, CDCl3) δ (ppm): 196.4, 196.4, 192.2, 163.0, 149.8, 131.9, 127.2, 124.6, 114.1, 50.6, 40.8, 32.1, 28.9, 27.6. HRMS (ESI-TOF) calcd. for C23H26NO5 : [M+H]+: 396.1733; found: 396.1763.
Results and Discussion
Since our interest was focused on the development of a green synthetic methodology for the synthesis of a selected group of 1,8-dioxo-octahydroxanthenes, the reaction of dimedone with benzaldehyde was used as a model reaction in order to find the best reaction conditions (Table 1).
The use of solvents like acetonitrile and dimethyl sulfoxide gave the tetraketone 4a in very poor yields (entries 2 and 4). On the other hand, ethanol, THF and water afforded the tetraketone 4a in good yields; however formation of 3a was not detected (TLC) (entries 1, 3 and 5). As reported by Yu and co-workers,(55) the formation of 4a can be explained by the fact that these solvents may help in the enolization of dimedone by making hydrogen bonds with the OH of the enol form of 2 and, thus, it increases the nucleophilic character of the methylene carbon (C-2). In addition, water and ethanol can also increase the electrophilic character of the carbonyl carbon of benzaldehyde by forming hydrogen bonds with the carbonyl oxygen.
It is known that the 1,8-dioxo-octahydroxanthenes can be obtained by intramolecular cyclization of 4a promoted by acid catalysts.(29,31,35) Therefore, acetic acid and other naturally occurring organic acids were examined for the obtention of 3a (Table 1, entries 6 to 13). Acetic acid afforded 3a with 65% yield, but a dilution of 50% decrease the yield to 58%. When 0.3 M solutions of oxalic, succinic, tartaric and citric acid were used as reaction media, yields above 77% were obtained for compound 3a and no tetraketone 4a was detected (Table 1 entries 8 to 11). However, similarly to entry 7, when the concentration of citric acid decreased the compound is formed in a low yield together with 4a. Interestingly, lemon juice also provided compound 3a in good yield. As shown in Figure 1, at the beginning of the reaction after benzaldehyde and 5,5-dimethyl-1,3-cyclohexanedione were added to aqueous citric acid (2 mL, 0.3M), a two-phase heterogeneous system was formed (Figure 1 left). After this, within the reaction progress, the formation of a white solid was observed. This precipitate product (Figure 1 right) could be easily filtrated and washed with water and aqueous ethanol. The same phenomenon was observed when the other aqueous naturally occurring organic acids and lemon juice were used.
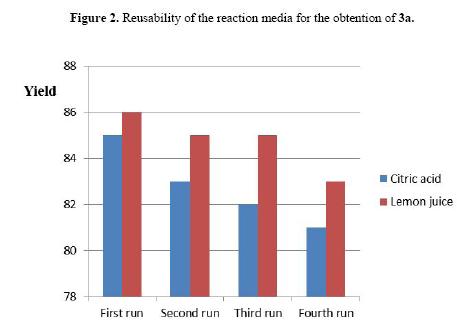
To study the reusability of the acidic media, we performed the same experiment described above by using lemon juice and 0.3 M citric acid (highest yields found, Table 1). After the reaction was completed the mixture was cooled to room temperature and the product was isolated by filtration. The recovered citric acid aqueous solution and lemon juice were reused as acidic media for three additional reactions to obtain the compound 3a. These experiments showed small decreases in the yield of 3a, which evidence the high reusability of this reaction media (Figure 2).
Additionally, using the best experimental conditions to perform the xanthene synthesis, indicated in entries 11 and 13 of Table 1, we examine the scope and generality of the citric acid and lemon juice-mediated synthesis by employing various aromatic aldehydes (Table 2). First, the reaction was carried out by using a series of different aldehydes, 5,5-dimethylcyclohexane-1,3-dione and lemon juice as solvent (Table 2, entries 2, 6 and 8). Although lemon juice was proved to be capable of promoting the reaction with benzaldehyde, it was found that when both electron-rich and electron-deficient aromatic aldehydes were used, the reaction was not completed and a mixture of the desired xanthene and tetraketone was obtained in low yields, together with starting materials (Table 2, entries 2, 6 and 8). Increasing the reaction time and temperature did not improve the yield of xanthenes and the lemon juice turned brown probably due to the decompositions of some components present in it. However, when the reaction was conducted in 0.3 M citric acid the xanthene derivatives 3b, 3f and 3h were obtained in good yields (Table 2, entries 2, 6 and 8). Following the same procedure, both electron-rich (Table 2, entries 3-5) and electron-deficient aromatic aldehydes (Table 2, entries 7 and 9) as well as heteroaromatic aldehydes (Table 2, entries 10 and 11) were converted into desired products in moderate to good yields. It is important to note here that just compounds 3i-3k were purified by recrystallization from ethanol and the other derivatives were obtained as pure compounds after washing with water and aqueous ethanol.
A plausible mechanism for the formation of compounds 3a-k is given in Scheme 1. First, the formation of the Knoevenagel adduct 6 promoted by citric acid takes place by the reaction of the enolic form of dimedone 5 with the aromatic aldehyde. Then 6 may further undergo Michael addition with another molecule of dimedone, in its enol form, to yield intermediate 7, which after a dehydration reaction gives compounds 3.
Conclusions
We described a simple, practical, economically viable and environmentally benign one-pot method for the synthesis of 9-aryl-1,8-dioxo-octahydroxanthenes. All reactions were performed in aqueous citric acid under mild conditions and using aromatic aldehydes containing both electro-donating and electron-withdrawing groups yielding the desired compounds in good yields.
Compared with the previously reported methods, our green protocol has the following advantages: mild reaction conditions, all products could be easily isolated by filtration avoiding the use of toxic organic solvents for the workup and thus minimizing waste, easy purification by recrystallization in ethanol when required (bypassing the need for costly column chromatography), reusability of the reaction media and the use of low-cost and readily available citric acid to promote the reaction.
Acknowledgements
The authors are thankful to the Universidad Nacional de Colombia for the support of this work.
References
1. Casillas, L.K.; Townsend, C.A. Total Synthesis of O-Methylsterigmatocystin Using N-Alkylnitrilium Salts and Carbonyl-Alkene Interconversion in a New Xanthone Synthesis, J. Org. Chem. 1999. 64: 4050-4059. [ Links ]
2. Piettre, A.; Chevenier, E.; Massardier, C.; Gimbert, Y.; Greene, A.E. Synthetic Approach to Hypoxyxylerone, Novel Inhibitor of Topoisomerase I, Org. Lett. 2002. 4: 3139-3142. [ Links ]
3. Suzuki, Y.; Fukuta, Y.; Ota, S.; Kamiya, M.; Sato, M. Xanthone Natural Products via N-Heterocyclic Carbene Catalysis: Total Synthesis of Atroviridin, J. Org. Chem. 2011. 76: 3960-3967. [ Links ]
4. Eguchi, T.; Kondo, K.; Kakinuma, K.; Uekusa, H.; Ohashi, Y.; Mizoue, K.; Qiao, Y.-F. Unique Solvent-Dependent Atropisomerism of a Novel Cytotoxic Naphthoxanthene Antibiotic FD-594, J. Org. Chem. 1999. 64: 5371-5376. [ Links ]
5. Konno, F.; Ishikawa, T.; Kawahata, M.; Yamaguchi, K. Concise Synthesis of Arnottin I and (-)-Arnottin II, J. Org. Chem. 2006. 71: 9818-9823. [ Links ]
6. Rarig, R.-A.F.; Tran, M.N.; Chenoweth, D.M. Synthesis and Conformational Dynamics of the Reported Structure of Xylopyridine A, J. Am. Chem. Soc. 2013. 135: 9213-9219. [ Links ]
7. Lambert, R.W.; Martin, J.A.; Merrett, J.H.; Parkes, K.E.B.; Thomas, G.J. Pyrimidine nucleosides, PCT Int. Appl. WO 9706178, 1997. [ Links ]
8. Hideo, T.; Teruomi, J. 1-Benzopyrano[2,3-b]xanthene derivative and its preparation, Jpn. Pat. 56005480, 1981. [ Links ]
9. Poupelin, J.P.; Saint-Ruf, G.; Foussard-Blanpin, O.; Narcisse, G.; Uchida-Ernouf, G.; Lacroix, R. Synthesis and antiinflammatory properties of bis(2-hydroxy-1-naphthyl)methane derivatives. I. Monosubstituted derivatives, Eur. J. Med. Chem. 1978. 13: 67-71. [ Links ]
10. Callan, J.F.; Silva, A.P.D.; Magri, D.C. Luminescent sensors and switches in the early 21st century, Tetrahedron 2005. 61: 8551-8588. [ Links ]
11. Hamblin, M.R.; Hasan, T. Photodynamic therapy: a new antimicrobial approach to infectious disease?, Photochem. Photobiol. Sci. 2004. 3: 436-450. [ Links ]
12. Renno, R.Z.; Miller, J.W. Photosensitizer delivery for photodynamic therapy of choroidal neovascularization, Adv. Drug Deliv. Rev. 2001. 52: 63-78. [ Links ]
13. Banerjee, A.; Mukherjee, A.K. Chemical Aspects of Santalin as a Histological Stain, Biotech. Histochem. 1981. 56: 83-85. [ Links ]
14. Knight, C.G.; Stephens, T. Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles., Biochem. J. 1989. 258: 683-687. [ Links ]
15. Ahmad, M.; King, T.A.; Ko, D.-K.; Cha, B.H.; Lee, J. Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases, J. Phys. D: Appl. Phys. 2002. 35: 1473-1476. [ Links ]
16. He, F.; Li, P.; Gu, Y.; Li, G. Glycerol as a promoting medium for electrophilic activation of aldehydes: catalyst-free synthesis of di(indolyl)methanes, xanthene-1,8(2H)-diones and 1-oxo-hexahydroxanthenes, Green Chem. 2009. 11: 1767-1773. [ Links ]
17. Kantevari, S.; Bantu, R.; Nagarapu, L. HClO4-SiO2 and PPA-SiO2 catalyzed efficient one-pot Knoevenagel condensation, Michael addition and cyclo-dehydration of dimedone and aldehydes in acetonitrile, aqueous and solvent free conditions: Scope and limitations, J. Mol. Catal. A: Chem. 2007. 269: 53-57. [ Links ]
18. Lü, H.-Y.; Li, J.-J.; Zhang, Z.-H. ZrOCl2·8H2O: a highly efficient catalyst for the synthesis of 1,8-dioxo-octahydroxanthene derivatives under solvent-free conditions, Appl. Organometal. Chem. 2009. 23: 165-169. [ Links ]
19. Shakibaei, G.I.; Mirzaei, P.; Bazgir, A. Dowex-50W promoted synthesis of 14-aryl-14H-dibenzo[a,j]xanthene and 1,8-dioxo-octahydroxanthene derivatives under solvent-free conditions, Appl. Catal., A: Gen. 2007. 325: 188-192. [ Links ]
20. Verma, G.K.; Raghuvanshi, K.; Verma, R.K.; Dwivedi, P.; Singh, M.S. An efficient one-pot solvent-free synthesis and photophysical properties of 9-aryl/alkyl-octahydroxanthene-1,8-diones, Tetrahedron 2011. 67: 3698-3704. [ Links ]
21. Shirini, F.; Khaligh, N.G. Succinimide-N-sulfonic acid: An efficient catalyst for the synthesis of xanthene derivatives under solvent-free conditions, Dyes Pigm. 2012. 95: 789-794. [ Links ]
22. Maleki, B.; Gholizadeh, M.; Sepehr, Z. 1,3,5-Trichloro-2,4,6-Triazinetrion: A Versatile Heterocycle for the One-Pot Synthesis of 14-Aryl- or Alkyl -14H-Dibenzo[a,j]xanthene, 1,8-Dioxooctahydroxanthene and 12-Aryl-8,9,10,12-Tetrahydrobenzo[a]xanthene-11-one Derivatives under Solvent-Free Conditions, Bull. Korean Chem. Soc. 2011. 32: 1697-1702. [ Links ]
23. Zolfigol, M.A.; Khakyzadeh, V.; Moosavi-Zare, A.R.; Zare, A.; Azimi, S.B.; Asgari, Z.; Hasaninejad, A. Preparation of various xanthene derivatives over sulfonic acid functionalized imidazolium salts (SAFIS) as novel, highly efficient and reusable catalysts, C. R. Chim. 2012. 15: 719-736. [ Links ]
24. Dabiri, M.; Baghbanzadeh, M.; Arzroomchilar, S. 1-methylimidazolium triflouroacetate ([Hmim]TFA): An efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines, Catal. Commun. 2008. 9: 939-942. [ Links ]
25. Fang, D.; Gong, K.; Liu, Z.-L. Synthesis of 1,8-Dioxo-octahydroxanthenes Catalyzed by Acidic Ionic Liquids in Aqueous Media, Catal. Lett. 2009. 127: 291-295. [ Links ]
26. Rad-Moghadam, K.; Azimi, S.C. Mg(BF4)2 doped in [BMIm][BF4]: A homogeneous ionic liquid-catalyst for efficient synthesis of 1,8-dioxo-octahydroxanthenes, decahydroacridines and 14-aryl-14H-dibenzo[a,j]xanthenes, J. Mol. Catal. A: Chem. 2012. 363-364: 465- 469. [ Links ]
27. Zare, A.; Moosavi-Zare, A.R.; Merajoddin, M.; Zolfigol, M.A.; Hekmat-Zadeh, T.; Hasaninejad, A.; Khazaei, A.; Mokhlesi, M.; Khakyzadeh, V.; Derakhshan-Panah, F.; Beyzavi, M.H.; Rostami, E.; Arghoon, A.; Roohandeh, R. Ionic liquid triethylamine-bonded sulfonic acid {[Et3N-SO3H]Cl} as a novel, highly efficient and homogeneous catalyst for the synthesis of ß-acetamido ketones, 1,8-dioxo-octahydroxanthenes and 14-aryl-14H-dibenzo[a,j]xanthenes, J. Mol. Liq. 2012. 167: 69-77. [ Links ]
28. Bigdeli, M. Clean synthesis of 1,8-dioxooctahydroxanthenes promoted by DABCO-bromine in aqueous media, Chin. Chem. Lett. 2010. 21: 1180-1182. [ Links ]
29. Darviche, F.; Balalaie, S.; Chadegani, F. Diammonium Hydrogen Phosphate as a Neutral and Efficient Catalyst for Synthesis of 1,8-Dioxo-octahydroxanthene Derivatives in Aqueous Media, Synth. Commun. 2007. 37: 1059-1066. [ Links ]
30. Kamble, S.; Rashinkar, G.; Kumbhar, A.; Salunkhe, R. Hydrotrope induced synthesis of 1,8-dioxo-octahydroxanthenes in aqueous medium, Green Chem. Lett. Rev. 2012. 5: 101-107. [ Links ]
31. Das, B.; Thirupathi, P.; Reddy, K.R.; Ravikanth, B.; Nagarapu, L. An efficient synthesis of 1,8-dioxo-octahydroxanthenes using heterogeneous catalysts, Catal. Commun. 2007. 8: 535-538. [ Links ]
32. Javid, A.; Heravi, M.M.; Bamoharram, F.F. One-Pot Synthesis of 1,8-Dioxo-octahydroxanthenes Utilizing Silica-Supported Preyssler Nano Particles as Novel and Efficient Reusable Heterogeneous Acidic Catalyst, E-J. Chem. 2011. 8: 910-916. [ Links ]
33. Niknam, K.; Panahi, F.; Saberi, D.; Mohagheghnejad, M. Silica-Bonded S-Sulfonic Acid as Recyclable Catalyst for the Synthesis of 1,8-Dioxo-decahydroacridines and 1,8-Dioxo-octahydroxanthenes, J. Heterocyclic Chem. 2010. 47: 292-300. [ Links ]
34. Oskooie, H.A.; Tahershamsi, L.; M.Heravi, M.; Baghernejad, B. Cellulose Sulfonic Acid: An Efficient Heterogeneous Catalyst for the Synthesis of 1, 8-Dioxo-octahydroxanthenes, E-J. Chem. 2010. 7: 717-720. [ Links ]
35. Pramanik, A.; Bhar, S. Alumina-sulfuric acid catalyzed eco-friendly synthesis of xanthenediones, Catal. Commun. 2012. 20: 17-24. [ Links ]
36. Das, P.; Dutta, A.; Bhaumik, A.; Mukhopadhyay, C. Heterogeneous ditopic ZnFe2O4 catalyzed synthesis of 4H-pyrans: further conversion to 1,4-DHPs and report of functional group interconversion from amide to ester, Green Chem. 2014. 14: 1426-1435. [ Links ]
37. Shaterian, H.; Rigi, F. An efficient synthesis of quinazoline and xanthene derivatives using starch sulfate as a biodegradable solid acid catalyst, Res. Chem. Intermed. 2013. 1-18. [ Links ]
38. Khazaei, A.; Moosavi-Zare, A.R.; Mohammadi, Z.; Zare, A.; Khakyzadeh, V.; Darvishi, G. Efficient preparation of 9-aryl-1,8-dioxo-octahydroxanthenes catalyzed by nano-TiO2 with high recyclability, RSC Adv. 2013. 3: 1323-1326. [ Links ]
39. Tu, S.; Gao, Y.; Miao, C.; Zhu, S.; Li, T.; Zhang, X.; Shi, D. The Reaction of Aromatic Dialdehyde with Dimedone Under Microwave Irradiation, Synth. Commun. 2004. 34: 2617-2622. [ Links ]
40. Dadhania, A.N.; Patel, V.K.; Raval, D.K. Catalyst-free sonochemical synthesis of 1,8-dioxo-octahydroxanthene derivatives in carboxy functionalized ionic liquid, C. R. Chim. 2012. 15: 378-383. [ Links ]
41. Jin, T.-S.; Zhang, J.-S.; Wang, A.-Q.; Li, T.-S. Ultrasound-assisted synthesis of 1,8-dioxo-octahydroxanthene derivatives catalyzed by p-dodecylbenzenesulfonic acid in aqueous media, Ultrason. Sonochem. 2006. 13: 220-224. [ Links ]
42. Rostamizadeh, S.; Amani, A.M.; Mahdavinia, G.H.; Amiri, G.; Sepehrian, H. Ultrasound promoted rapid and green synthesis of 1,8-dioxo-octahydroxanthenes derivatives using nanosized MCM-41-SO3H as a nanoreactor, nanocatalyst in aqueous media, Ultrason. Sonochem. 2010. 17: 306-309. [ Links ]
43. Venkatesan, K.; Pujari, S.S.; Lahoti, R.J.; Srinivasan, K.V. An efficient synthesis of 1,8-dioxo-octahydro-xanthene derivatives promoted by a room temperature ionic liquid at ambient conditions under ultrasound irradiation, Ultrason. Sonochem. 2008. 15: 548-553. [ Links ]
44. Mulakayala, N.; Pavan Kumar, G.; Rambabu, D.; Aeluri, M.; Basaveswara Rao, M.V.; Pal, M. A greener synthesis of 1,8-dioxo-octahydroxanthene derivatives under ultrasound, Tetrahedron Lett 2012. 53: 6923-6926. [ Links ]
45. Berovic, M.; Legisa, M., Citric acid production, in: Biotechnology Annual Review, M.R. El-Gewely (Ed.) Amsterdam, Elsevier, pp. 303-343, 2007. [ Links ]
46. Belitz, H.-D.; Grosch, W., Food Chemistry, Second edition ed., Heidelberg, Springer-Verlag, pp., 1999. [ Links ]
47. Kristiansen, B.; Mattey, M.; Linden, J., Citric Acid Biotechnology, London, Taylor & Francis Ltd, pp., 2002. [ Links ]
48. Rohokale, S.; Kote, S.; Deshmukh, S.; Thopate, S. Natural organic acids promoted Beckmann rearrangement: Green and expeditious synthesis of amides under solvent-free conditions, Chem. Pap. 2014. 68: 575-578. [ Links ]
49. Thopate, S.R.; Kote, S.R.; Rohokale, S.V.; Thorat, N.M. Citric acid catalysed Beckmann rearrangement, under solvent free conditions, J. Chem. Res. 2011. 35: 124-125. [ Links ]
50. Vasconcelos, A.d.; Oliveira, P.S.; Ritter, M.; Freitag, R.A.; Romano, R.L.; Quina, F.H.; Pizzuti, L.; Pereira, C.M.P.; Stefanello, F.M.; Barschak, A.G. Antioxidant Capacity and Environmentally Friendly Synthesis of Dihydropyrimidin-(2H)-ones Promoted by Naturally Occurring Organic Acids, J. Biochem. Mol. Toxicol. 2012. 26: 155-161. [ Links ]
51. Ghorbani-Choghamarani, A.; Taghipour, T. Green and One-Pot Three-Component Synthesis of 2,3-Dihydroquinazolin- 4(1H)-Ones Promoted by Citric Acid as Recoverable Catalyst in Water, Lett. Org. Chem. 2011. 8: 470-476. [ Links ]
52. Enugala, R.; Nuvvula, S.; Kotra, V.; Varala, R.; Adapa, S.R. Green Approach for the Efficient Synthesis of Quinolines Promoted by Citric Acid, Heterocycles 2008. 75: 2523-2533. [ Links ]
53. Martinez Gomez, S.M.; Alzate Sanchez, D.M.; Rodríguez-Córdoba, W.; Sierra, C.A.; Ochoa-Puentes, C. Competitive One-Pot Reactions: Simultaneous Synthesis of Decahydroacridine-1,8-diones and 1,8-Dioxo-octahydroxanthenes and Photophysical Characterization, Synth. Commun. 2014. 44: 648-659. [ Links ]
54. Rodríguez-Córdoba, W.; Sierra, C.A.; Ochoa-Puentes, C.; Lahti, P.M.; Peon, J. Photoinduced Energy Transfer in Bichromophoric Pyrene-PPV Oligomer Systems: The Role of Flexible Donor-Acceptor Bridges, J. Phys. Chem. B 2012. 116: 3490-3503. [ Links ]
55. Yu, J.-J.; Wang, L.-M.; Liu, J.-Q.; Guo, F.-L.; Liu, Y.; Jiao, N. Synthesis of tetraketones in water and under catalyst-free conditions, Green Chem. 2010. 12: 216-219. [ Links ]