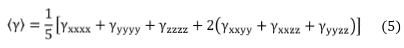Introduction
In the last few years the engineering of organic crystalline compound has become an important area of study and has attracted great interest from research centers [1], motivated by significant values of the nonlinear optical properties of these crystals, for instance, chalcone derivatives [2]. Nonlinear optics devices operate with high-speed information [3], as ultra-short pulse lasers, photonic devices, optical modulators, and others with applications in the areas of health and medicine [4, 5]. The advantages of organic crystals that have attracted much attention come from their ease of manipulation of the molecular structure and the synthetic flexibility regarding the change of substituent groups [6, 7]. The combination of the high nonlinearity characteristic of some organic compounds and the versatility of synthetic routes leads to maximization of the nonlinear optical properties [8]. Among the organic materials, the chalcones are known as promising compounds, since their derivatives have several biological properties that have been used as prototypes for new drugs [9]. Biosynthesis of flavonoids chalcones have been applied in several activities such as: antitumor [10], anti-inflammatory [11], antibacterial [12], antifungal [13], among others.
The chalcones consist of two aromatic rings joined by an unsaturated system so that their structure almost always acquires a linear or almost planar conformation [14]. Organic molecules with n-conjugated structures and donor and/or acceptor groups are compounds of great interest in the area of nonlinear optics [15]. The change of the substituent groups bond to the aromatic rings leads the chalcone molecules to acquire specific properties due the change of electronic distribution of the compound, hence new properties can be explored with each new substituent being added or substituted to the compound [16].
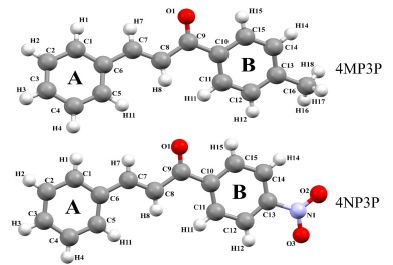
The purpose of the present paper is to study the nonlinear optical properties of two chalcone derivatives: (E)-1-(4-methylphenyl)-3-phenylprop-2-en-1-one (4MP3P) and (E)-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one (4NP3P), with formulas C16H14O and C15H11O3, respectively. The structure 4MP3P was synthesized by Toda et al. [15] and the structure 4NP3P by Jing et al. [16]. These compounds differ by the substituent at position 4 of the phenyl ring (B) as shown in Figure 1.
The electrical parameters of these compounds were calculated at DFT/ CAM-B3LYP/6-311+G(d) level in the presence of several solvent media that are modeled by the Polarizable Continuum Model (PCM). The static and dynamic (1064 nm) molecular parameters as the dipole moment, linear polarizability, first and second hyperpolarizability were studied as function of the value of the solvent dielectric constant value. Also, the frontiers molecular orbital, HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital), and the respective gap energies in various solvents were calculated. The geometrical behavior of the compounds as the chemical bond angles, torsion angles, and partial charges distribution were also studied.
Materials and Methods
Computational procedures
Organic solvents belong to a class of liquid and volatile compounds and have the function of solubilizing, extracting, treating, and facilitating a chemical reaction among other functions. This group of compounds is divided into: polar and nonpolar solvents. Polar protic solvents are characterized by the presence of hydrogen bonded to electronegative elements, usually, atoms of O, N, and F, which can lead to the formation of hydrogen bonds [17] and polar aprotic solvents are characterized by the absence of electronegative atoms so that only bonds between carbon and hydrogen atoms are present. The values of the dielectric constant and of the dipole moments for polar solvents are higher than those for the nonpolar solvents [18]. The dielectric constant (e) of a solvent medium is a good indicator of the ability to accommodate a charge separation that increases with the values of the dipole moment and with the polarizability of the molecule [19].
In this work the quantitative concept of the solvent polarity was considered through the normalized transition energy (E T N ) scale of Dimroth and Reichardt [19, 20]. The ET N-value is based on the transition energy to the solvatochromic absorption band for the greatest wavelenght of the nitrile betaine pyridynium dye. Table 1 shows a list of the solvent media, here considered with the respective values of E T N and e. Reactions involving charge separation in the E T N-scale advance more slowly in polar aprotic solvents due to the small dipole moments and the absence of hydrogen bonds which make them few effective in the development of the separation and the stabilization of charges when compared to the polar protic solvents[19, 21].
Table 1: Polarity of several solvent media following ET N-scale [22].
| Solvents | £ | Type | |
|---|---|---|---|
| Water | 1.000 | 78.355 | Protic |
| Methanol | 0.762 | 32.613 | Protic |
| Ethanol | 0.654 | 24.852 | Protic |
| DMSO | 0.444 | 46.826 | Aprotic |
| Acetone | 0.355 | 20.493 | Aprotic |
| Dichloroethane | 0.327 | 10.125 | Aprotic |
| Chloroform | 0.259 | 4.711 | nonpolar |
| Tetrahydrofuran | 0.207 | 7.430 | Aprotic |
| Heptane | 0.012 | 1.911 | nonpolar |
The first two parameters are dimensionless.
Here the solvent chloroform must be considered nonpolar because the value of its dielectric constant s is less than 5.
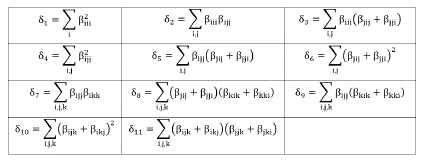
The total dipole moment and the average linear polarizability were calculated from the components x, y, and z, through the expressions:
The first hyperpolarizabilities considered concerned here were the parallel components to the direction of dipole moment (taken as z-direction) given by,
and the Hyper-Rayleigh Scattering first hyperpolarizability (β HRS ) defined by,
where the laboratory system of reference is adopted as X, Y, and Z coordinates. The X-direction is assumed as the fundamental light beam propagation, polarized in the Z-direction. The (β 2 zzz) and (β 2 xzz) are macroscopic averages calculated from the first hyperpolarizability components (β tJk ) through the expressions,
in which the coefficients δn are defined in Table 2.
Using the Kleymann symmetry, the average value of the second hyperpolarizability (y) can be calculated through the following expression,
The linear and nonlinear optical (NLO) parameters of the molecules of chalcone derivatives in several solvent media were calculated using the PCM/DFT/CAM-B3LYP/6-311+G(d) level with the Gaussian 09 software package.
Results and Discussion
Solvent efects on the molecular properties
In this section, the similarities between the molecular geometry of 4MP3P and 4NP3P, obtained via X-ray diffraction [15, 16], and the theoretical results in several solvent media are analyzed through the root mean square deviation (RMSD) of the overlap of the two structures. As can be seen, in Table 3, the value of RMSD is 0.156 a.u. (0.217 a.u.) for gas phase of 4MP3P (4NP3P) and this value presents small changes when the solvent dielectric constant increases, until reaching the value 0.167 a.u. (0.295 a.u.), therefore the effects of solvent media on the molecular geometry is more significant for 4NP3P. Figure 2 shows a schematic representation of the overlap of 4MP3P and 4NP3P molecular structure determined by X-ray (in blue) and in water (in red), where the phenyl ring (A) was used as anchorage for the molecular structures. The H-atoms were disregarded in view of their uncertainties in X-ray position refinement.
Table 3 RMSD (a.u.) and Gap energies (eV) of 4MP3P and 4NP3P.
| Solvent | f | RMSD 4MP3P | RMSD 4NP3P | Gap (eV) 4MP3P | Gap (eV) 4NP3P |
| Gas-phase | 1.00 | 0.156 | 0.217 | 6.636 | 6.094 |
| Heptane | 1.91 | 0.158 | 0.285 | 6.587 | 5.980 |
| Chloroform | 4.71 | 0.162 | 0.292 | 6.537 | 5.886 |
| Tetrahydrofuran | 7.43 | 0.163 | 0.293 | 6.520 | 5.859 |
| Dichloroethane | 10.13 | 0.164 | 0.294 | 6.511 | 5.846 |
| Acetone | 20.49 | 0.165 | 0.295 | 6.499 | 5.829 |
| Ethanol | 24.85 | 0.166 | 0.295 | 6.496 | 5.825 |
| Methanol | 32.61 | 0.166 | 0.295 | 6.494 | 5.822 |
| DMSO | 46.71 | 0.166 | 0.295 | 6.491 | 5.819 |
| Water | 78.36 | 0.167 | 0.295 | 6.489 | 5.815 |

Figure 2 Overlap of the theoretical molecular structures of the compounds (4MP3P and 4NP3P) in water (red) and that determined by X-ray diffraction (blue).
Table 4 shows the (O1-C9-C8-C7), (C8-C9-C10-C11), (C8-C9-C10-C15), (C8-C7-C6-C5), (C8-C7-C6-C5), and (01-C9-C10-C15) torsion angles for various solvents and for the gas-phase. As can be seen, the torsion angles present a small variations in all solvent media, independent of the solvent polarity or the protic or aprotic character for both compunds 4MP3P and 4NP3P.
Table 4 Geometrical parameters related to torsion angle (°).
| 01-C9-C8-C7 | C8-C9-C10-C11 | C8-C9-C10-C15 | C8-C7-C6-C1 | C8-C7-C6-C5 | 01-C9-C10-C15 | ||||||||
| 4MP3P | 4NP3P | 4MP3P | 4NP3P | 4MP3P | 4NP3P | 4MP3P | 4NP3P | 4MP3P | 4NP3P | 4MP3P | 4NP3P | ||
| X-Ray | 17.83 | 0.54 | 6.84 | -5.8 | -176.07 | 176,00 | -179.29 | -171.29 | 2.25 | 8.14 | 3.97 | -4.37 | |
| Gas-Phase | 5.86 | 5.86 | 14.09 | 20.69 | -167.3 | -160.95 | -176.61 | -177.11 | 3.66 | 2.97 | 12,00 | 18.22 | |
| Heptane | 6.18 | -5.84 | 15,00 | -22.4 | -166.42 | 159.3 | -176.95 | 176.91 | 3.19 | -3.17 | 12.87 | -19.85 | |
| Chloroform | 6.44 | -5.7 | 15.79 | -23.51 | -165.65 | 158.2 | -177.27 | 177.29 | 2.84 | -2.75 | 13.62 | -20.98 | |
| Tetrahydrofuran | 6.5 | -5.66 | 16.03 | -23.83 | -165.41 | 157.88 | -177.37 | 177.56 | 2.74 | -2.47 | 13.85 | -21.31 | |
| Dichloroethane | 6.52 | -5.65 | 16.14 | 28.98 | -165.29 | 157.72 | -177.42 | 177.64 | 2.69 | -2.38 | 13.96 | -21.48 | |
| Acetone | 6.55 | -5.62 | 16.32 | -24.23 | -165.1 | 157.45 | -177.51 | 177.93 | 2.59 | -2.07 | 14.14 | -21.75 | |
| Ethanol | 6.56 | -5.61 | 16.36 | -24.28 | -165.06 | 157.4 | -177.53 | 178,00 | 2.57 | -2.01 | 14.17 | -21.8 | |
| Methanol | 6.56 | -5.61 | 16.4 | -24.33 | -165.02 | 157.35 | -177.55 | 178.07 | 2.55 | -1.92 | 14.21 | -21.86 | |
| DMSO | 6.57 | -5.59 | 16.44 | -24.39 | -164.97 | 157.29 | -177.57 | 178.15 | 2.53 | -1.84 | 14.25 | -21.92 | |
| Water | 6.57 | -5.58 | 16.49 | -24.44 | -164.93 | 157.23 | -177.59 | 178.23 | 2.51 | -1.76 | 14.29 | -21.98 | |
The results of the solvent torsion angles as compared with the gas-phase present a small variation in their absolute values and for 4NP3P a signal inversion occurs. In addition, in Table 4 the X-ray results for the torsion angles for the compounds in crystalline phase are presented. Comparing these angles with the results obtained in gas-phase one can see that greater differences occur for 4NP3P, whose variations can reach 27o.
Results for the angles (O1-C9-C8), (C8-C9-C10), (C8-C7-C6) for both compounds and the angles (N1-C13-C14) and (N1-C13-C12) for 4NP3P, and the angles (C16-C13-C14) and (C16-C13-C12) for 4MP3P are presented in Table 5. As can be seen, the results for these angles presents no significative variation caused by the presence of the solvent media.
The asymmetric distribution of electrons in the chemical bonds of the molecular compounds can be visualized by the partial charges calculations in gas-phase. For 4MP3P the total charges of the groups (C4-C5-C6-C7-C8-C9-C16-H3-H4-H5-H6-H12-H13-H14), (C10-C11-C12-C13-C14-C15-H7-H8-H9-H10-H11), and (C1-C2-C3-H1-H2-O1) are 0.012 e, 0.148 e and -0.159 e, respectively. For 4NP3P the total charges of the (C10-C15-H15-C14-H14-C13-C12-H12-C11-H11), (C1-H1-C2-H2-C3-H3-C4-H4-C5-H5-C6), (C7-H7-C8-H8-C9-H9-O1), and ( N1-O2-O3) are 0.170 e, 0.152 e, -0.101 e and -0.221 e, respectively. The transfer of charge is greater for the polar solvent media than that for the nonpolar solvent (ε<5).
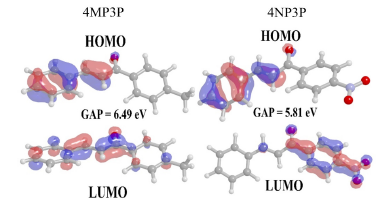
The gap energy (EG) calculated from the difference between the HOMO and LUMO energies is also shown in Table 3 for several solvent media. As can be noted, the substituent change, from CH3 (4MP3P) by the NO2 (4NP3P), causes a decreasing (~11%) in the gap energy in all solvent media.
Figure 3 shows the HOMO and LUMO orbitals for 4MP3P and 4NP3P in water, presenting the lowest values of gap energy. Also it shows that the smaller the value of gap energy the better the nonlinear optical properties of the compound [7]. Both HOMO and LUMO present π-bonds.
Table 6 shows the DFT/CAM-B3LYP/6-311+G(d) results for the dipole moment (µ) in debye units (D) for both compounds in the gas-phase and in various solvents. As can be seen the p-value increases with the increasing of the µ -value. For 4MP3P (4NP3P) the dipole moment increases 48.5% (23.6%) as compared with the gas-phase. As example, in water the substituent changes from 4-methylphenyl to 4-nitrophenyl increases the µ -value of 62.5%, evidencing a greater negative charge transfer for the NO2 group.
Table 6 Dipole moment values (D) for 4MP3P and 4NP3P in several solvent media.
| Solvent | ε | 4MP3P | 4NP3P |
| Gas-phase | 1 | 3.34 | 6.52 |
| Heptane | 1.91 | 3.84 | 7.12 |
| Chloroform | 4.71 | 4.39 | 7.64 |
| Tetrahydrofuran | 7.43 | 4.58 | 7.79 |
| Dichloroethane | 10.13 | 4.68 | 7.87 |
| Acetone | 20.49 | 4.84 | 7.98 |
| Ethanol | 24.85 | 4.86 | 8.00 |
| Methanol | 32.61 | 4.89 | 8.02 |
| DMSO | 46.7 | 4.92 | 8.05 |
| Water | 78.36 | 4.96 | 8.06 |
The behavior of the static values of the average linear polarizability (<α(0,0)>) first hyperpolarizability (<β||(0,0,0)>) and average second hyperpolarizability (<γ(0,0,0,0)>) as function of ε-values for both compounds are shown in the Table 7. As can be seen, all absolute values of these parameters increase when the s-values for both compounds also increase, the result being greater for 4NP3P. Comparing the absolute values of these parameters for 4MP3P with those for 4NP3P,we can see that the static linear polarizability exhibits the smallest variation (~3%) and the absolute values of the parallel first hyperpolarizability present the greatest variation (more than 150%). So, the substituent change benefits the nonlinear optical properties of the compounds; this fact can be seen for the <γ(0,0,0,0)>-value that is greater (~20%) for 4NP3P than for 4MP3P, showing that accumulation of negative charges in the NO2 group favors the electron cloud distortion.
Table 7 Static values of the average linear polarizability (in 10 24 esu), first hyperpolarizability (in 1030 esu) and average second hyperpolarizability (in 1036 esu) for 4MP3P and 4NP3P.
| Solvent | 4MP3P | 4NP3P | 4MP3P | 4NP3P | 4MP3P | 4NP3P |
| α(0; 0) | α (0; 0) | (β||Z(0;0,0) | (β||Z(0;0,0) | γ||(0; 0,0,0) | γ||(0; 0,0,0) | |
| Gas-phase | 30.80 | 31.72 | -4.58 | -13.81 | 61.03 | 69.70 |
| Heptane | 34.37 | 35.46 | -7.19 | -21.11 | 91.54 | 108.10 |
| Chloroform | 38.14 | 39.40 | -10.69 | -29.60 | 131.53 | 158.49 |
| Tetrahydrofuran | 39.42 | 40.73 | -12.04 | -32.56 | 146.95 | 177.54 |
| Dichloroethane | 40.09 | 41.42 | -12.77 | -34.09 | 155.30 | 187.70 |
| Acetone | 41.11 | 42.46 | -13.92 | -36.41 | 168.51 | 203.57 |
| Ethanol | 41.29 | 42.65 | -14.13 | -36.84 | 170.98 | 206.51 |
| Methanol | 41.51 | 42.87 | -14.38 | -37.32 | 173.84 | 209.90 |
| DMSO | 41.72 | 43.09 | -14.62 | -37.80 | 176.69 | 213.29 |
| Water | 41.92 | 43.29 | -14.86 | -38.25 | 179.41 | 216.49 |

Figure 4 Static and dynamic HRS first-hyperpolarizabilities as function of the dielectric constant values.
The increasing of the α(0;0)-values is greater for the nonpolar solvents (~14%) than for the aprotic (~4%) and protic (~1.5%) solvents for both compounds. However, the increasing of the static absolute β-values and y-values are basically the same for both 4MP3P and 4NP3P, in protic solvents around of 5%, in aprotic solvents around of ~14% and for nonpolar solvents around 60%. These results show that for the nonpolar solvents the dielectric properties of the medium are the strongest characteristic to be considered, but for the aprotic and protic solvents considered here, despite the great increasing of values of the solvent dielectric constant between then, the effect on the electric parameters is small.
Table 8 shows the static and dynamic (ω=0.0428 a.u.) results for HRS hyperpolarizabilities (β HRS ) as function of the dielectric constant value; as can be seen, the β HRS (0;0,0)-value increases when the s-value also increases. The highest values of the static-β HRS occur in water 26.53-10-30 esu and 15.846 10-30 esu for 4NP3P and 4MP3P, respectively, indicating a significant difference of 67% due the substituent change at position 4 of the phenyl ring. The highest values of the dynamic β HRS (-2ω; ω, ω) occur in DMSO, namely 15.2M0-30 esu (4MP3P) and 38.145-10-30 esu (4NP3P).
Table 8 Static and dynamic values of the HRS first hyperpolarizabilities (in 10 30 esu) for 4MP3P and 4NP3P.

In Figure 4, for the two chalcone derivatives, the static and dynamic results for β HRS are plotted as function of the ε-values; as we can observe, the values for 4NP3P are greater than those of 4MP3P.
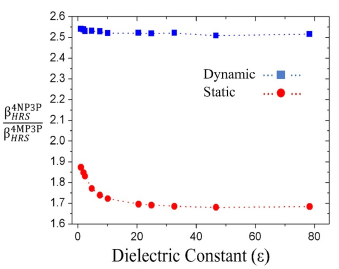
The β
HRS
-ratio between the compounds 4NP3P ( ) and 4MP3P
) and 4MP3P  as function of s for the dynamic (ω = 0.0428 a.u) and static case are shown in Figure 5. As can be seen, in the dynamic case, the β
HRS
-ratio is around 2.5 and for the static case the value lies between 1.7 and 1.9.
as function of s for the dynamic (ω = 0.0428 a.u) and static case are shown in Figure 5. As can be seen, in the dynamic case, the β
HRS
-ratio is around 2.5 and for the static case the value lies between 1.7 and 1.9.
Conclusions
We have reported the geometrical and nonlinear optical properties of two chalcone derivatives: (E)-1-(4-methylphenyl)-3-phenylprop-2-en-1-one (4MP3P) and (£)-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one (4NP3P), within the DFT/CAM-B3LYP/6-311+G(d) level. The effects of the various solvents on the electrical parameters of the two chalcone derivatives were considered via the Polarizable Continuum Model (PCM). The values of the dipole moment (p) increase with the increasing of the solvent dielectric constant values and this increasing is greater for 4MP3P than for 4NP3P. However, the substituent change of CH3 to NO2 increases the p-value for all solvents; as an example, in water this increase is of 62.5%, evidencing the greater negative charge transfer for the NO2 group. The static linear polarizability presents the smaller variation (~3%) due to the solvent medium presence; however, the absolute values of the parallel first hyperpolarizability present the greater variation (more than 150%). Therefore, the substituent change of CH3 (4MP3P) to NO2 (4NP3P) benefits the nonlinear optical properties of the compounds. The HRS hyperpolarizability (β HRS ) as function of the solvent dielectric constant value were calculated as in static and dynamic cases. The static β HRS -values also increase when the s-value increases. The highest values of the static β HRS occur in water 26.53-10-30esu and 15.846-10-30esu for 4NP3P and 4MP3P, respectively, indicating a significant difference of 67% due the substituent change.