Introduction
Avocado (Persea americana Mill. cv. Hass), Is a climacteric fruit which is characterized by its high nutritive value, having an important content of unsaturated fatty acids, fat soluble and water soluble vitamins, especially A and C. In addition, it has been pointed out that this fruit may be a functional food due to beneficial effects tested for human health (Jacobo-Velázquez & Hernández-Brenes, 2012).
This have allowed an increase in avocado consumption worldwide, mainly in countries such as the United States, France, Germany and Spain, which has resulted in an increase in planted areas, and in turn a variety of possibilities of uses as industrialized products (Duque, Londoño-Londoño, Álvarez, Paz, & Salazar, 2012).
Currently, the main industrialized avocado products, which are marketed as follows: pulp, as a base for spreadable products, both fresh and chilled or frozen, and oil. Avocado puree is the product, which has a greater volume of production when used as a base for spreadable products, mainly guacamole, a product composed of other ingredients such as tomato, onion, garlic, cilantro, jalapeno chili pepper, salt, acids and preservatives. Guacamole, is a popular seasoning in Mexico, and currently in the United States and Europe, as the basis of food called "Tex-Mex".
Guacamole, constitutes an oil-in-water colloidal system type emulsion, which is thermodynamically unstable, with its stability representing a critical and essential variable in the shelf-life of this food.
The physical destabilization of colloidal systems mechanisms or colloidal disruption, can be presented without variations in particle size, for example, when sedimentation or cremation and flocculation occur, or with particle size variation, including coagulation phenomena, coalescence, Ostwald maturation or phases inversion (Mirhosseini, Tan, Hamid, & Yusof, 2008).
Some authors have evaluated the effect of different compounds on the stability of oil-in-water emulsions as follows: globular protein sources (soybeans, peas and whey proteins) and the casein ratio in emulsions with soybean oil (Liang, Wong, Pham, & Tan, 2016), sodium caseinate use in emulsions based on peanut oil (Long et al., 2015), texturized whey protein incorporation into emulsions containing corn oil and butter oil (Ruttarattanamongkol, Nor Afizah, & Rizvi, 2015), sucrose esters and Tween 20.60 & 80, among others. In addition, the use of homogenization high speed and ultrasound in the preparation of stabilized emulsions with fenugreek gum (Kaltsa, Yanniotis, & Mandala, 2016), oil content, starch (starch octenyl sodium succinate) and pressure homogenization on the o/w emulsion stability ( Domian, Brynda-Kopytowska, & Oleksza, 2015).
The aim of this research was to evaluate the influence of composition and homogenization process on a colloidal system stability based on avocado (Persea americana Mill. cv. Hass) and other ingredients, for a potential use in aspersion drying process.
Materials and methods
Raw materials
We used fresh avocados (Persea americana Mill cv. Hass), from the municipality of Abejorral, Antioquia-Colombia, which were processed between 11 and 15 days of ripening, gum arabic (Master Gum FT), maltodextrin (between 18 and 20 dextrose equivalent), TBHQ (ter-butylhydroquinone), sodium chloride, food grade, onion (Allium cepa L.), garlic ((Allium sativum L.), bell pepper (Capsicum annuum L. ), tomato (Solanum lycopersicum L.), cilantro (Coriandrum sativum L.), lemon (Citrus aurantiifolia (Christm.) Swingle) and water.
Formulation and emulsion preparation
Emulsion formulations were standardized according to the avocado (Persea americana Mill cv. Hass), dry solids content (DSavocado), with a total acidity of 0.1% provided by lemon juice adjusted to 7% as citric acid. The other ingredients used were fixed in emulsions. Batches of 2000 g of emulsion were prepared, initially, avocado was homogenized with lemon juice and partial water content, the total solids being adjusted if required. Subsequently, a homogenized maltodextrin, gum arabic, TBHQ, NaCl, vegetable ingredients premixture and the rest of water was added. An Ultraturrax IKA homogenizer model TK50, USA, was used at a speed of 10.000 rpm.
Emulsions stability characterization
Z potential (ζ)
Emulsions zeta potential was measured using un Zetasizer Nano ZS90 (Malvern Instruments Ltd., Worcester, UK) (Rezvani, Schleining, & Taherian, 2012). Nano zetasizer calculates zeta potential by determining the electrophoretic mobility and then applying the Henry equation. To avoid multiple dispersion effects and air bubbles presence, emulsions were diluted with deionized water in an emulsion: water ratio (1: 100).
Stability index by spectral absorption (R)
It was determined from the absorbances ratio at two wavelengths (800 and 400 nm) (A800/A400) (Mirhosseini et al., 2008), using a UV-Visible Spectrophotometer (Thermo Scientific Evolution 60). Emulsion samples were diluted in water (1: 100) and triplicates were performed by emulsion. The R stability index is based on the light scattering properties, which is related to the average droplet size and wavelength.
Viscosity (µ)
It was determined using the rheometer (Brookfield DV-III Ultra, Brookfield Engineering Laboratories, Inc., USA), coupled with a Brookfield thermostated bath model TC-502, controlled temperature of 25° C, equipped with the spindle RV4 at a speed of 0.01 to 100 rpm, the viscosity being reported in cP at a speed of 100 rpm. A fixed emulsion volume was measured in a 600 mL beaker, and spindle depth was constant throughout the measurements (Mirhosseini, Tan, Hamid, Yusof, & Chern, 2009).
Peroxide index (PI)
It was determined by a spectrophotometric method, which relies on the peroxides ability to oxidize ferrous ions to ferric ions which react with various reagents that produce colored complexes. It was expressed as H2O2 milliequivalents per kg sample, using equation 1. Where Am and Ab, correspond to the sample and blank absorbance respectively, at a wavelength of 500 nm; M = calibration curve slope, W = sample weight (g); 2 = conversion factor to express as H2O2 meq, 55.84 = iron molecular weight and Vt = final volume (mL) of the reaction mixture (Equation 1).
Color
It was determined using an X-Rite spectrophotometer, model SP62 with D65 illuminant and 10° observer as reference. From the spectra reflection, we obtained the color coordinates for the ICD-La * b *, where L * is a luminosity indicator, a * (green chromaticity (-) to red (+)) and b * (blue chromaticity (-) to yellow (+)).
Particle size
Particle size distribution of the prepared emulsions, was determined using laser light diffraction in a Mastersizer 3000 (Malvern Instrument Ltd., Worcestershire, UK). Sample was added in 500mL of distilled water until a darkening value of 10 ± 1%, was obtained. The size distribution was calculated using the Mie theory by the refractive index of 1.52 (Millqvist-Fureby & Smith, 2007). Particle size was reported as D10, D50 y D90 percentiles.
Experimental design and statistical analysis
Response surface methodology was used with a composite central design to establish the main and combined effects of the independent variables as follows: (A) DSavocado (33.07% - 50.32%), (B) homogenization time (3 - 7 min) and (C) TBHQ concentration [TBHQ] (50 - 100 mg.kg-1) on dependent variables: ζ, L*, a*, b*, (, R, IP, D10, D50 and D90, obtaining 20 randomized experiments with six replicates at the central point. Table 1, describes the experimental design used. In addition, independent variables to optimize the emulsion proportion components in terms of dependent variables, was determined. We used a quadratic model (Equation 2), to analyze each dependent variable (Y) as a function of independent variables, where 〖β〗 _0, is a constant, βA, βB and βC is the linear coefficient for each factor; ΒA2, βB2 and βC2, is the quadratic coefficient of each factor; ΒAB, βAC and βBC is the product coefficient of the factor interactions.
The models adequacy was determined using the non-fit test and regression coefficient (R2). In addition, variance analysis (ANOVA), was performed with a significance level of 5%. The matrix of the experimental design, the results analysis and the procedure optimization was carried out using the software Statgraphics Centurion XVI.I(r).
Results and discussion
Tables 2 and 3, presents the mean values plus standard deviations of dependent variables, depending on the independent variables and the ANOVA. Figure 1, shows the response surface graphs of dependent variables, which were statistically significant (p<0.05).
Table 2 Quality attributes of avocado (Persea americana Mill. cv. Hass) based emulsions and other ingredients

Table 3 p-values for response surface models of avocado (Persea americana Mill. cv. Hass) based emulsions and other ingredients

*: Significant (p<= 0.05).
Z Potential (ζ)
As can be seen in Table 3, the potential-ζ did not show significant differences (p> 0.05) with respect to the considered independent variables. However, it was observed in all the experiments, absolute values of zeta potential greater than 25 mV (26.94 - 30.85mV) were performed, which indicates a good repulsive interaction or repulsive forces among colloidal particles, contributing to a good thermodynamic stability of the system (Mirhosseini et al., 2008; Rezvani et al., 2012).
Avocado (Persea americana Mill cv. Hass), is rich in mineral salts such as potassium, sodium, phosphorus, iron, copper and chlorine, which when are dissociated, produce an important ionic strength in continuous phase, whose ions are strongly adsorbed at the particles interface, which also have ionizable charge groups, producing or resulting in a strongly adsorbed negative charge at the particles interface. This situation also causes an electric potential to occur on the outskirts of this adsorbed layer (stern layer) associated with the zeta potential found. Furthermore, it contributes to the formation of a diffuse co-ions second layer distributed in a solution close to the interface. Both layers, form the known double-layer ion or Debye length ((-1).
Stability index by spectral absorption (R)
Stability index by spectral absorption (R) was significantly (p <0.05) influenced by DSavocado (A), observing the avocado increased content (increase in the system oil phase or dispersed phase), had a direct effect on R (Figure 1a), i.e., particles of larger diameter are produced in the emulsion. The prepared emulsions were stable, without phase separation, which was favored by the high viscosities of the evaluated formulations, which diminished from the difference between oil and water specific gravity (Dłuzewska, Stobiecka, & Maszewska, 2006). Similar results were reported by Gharibzahedi et al. (2012), where oil content had a direct effect on the R index in walnut emulsions. In addition, Mirhosseini et al. (2008), also reported the oil content had an inverse influence on R.
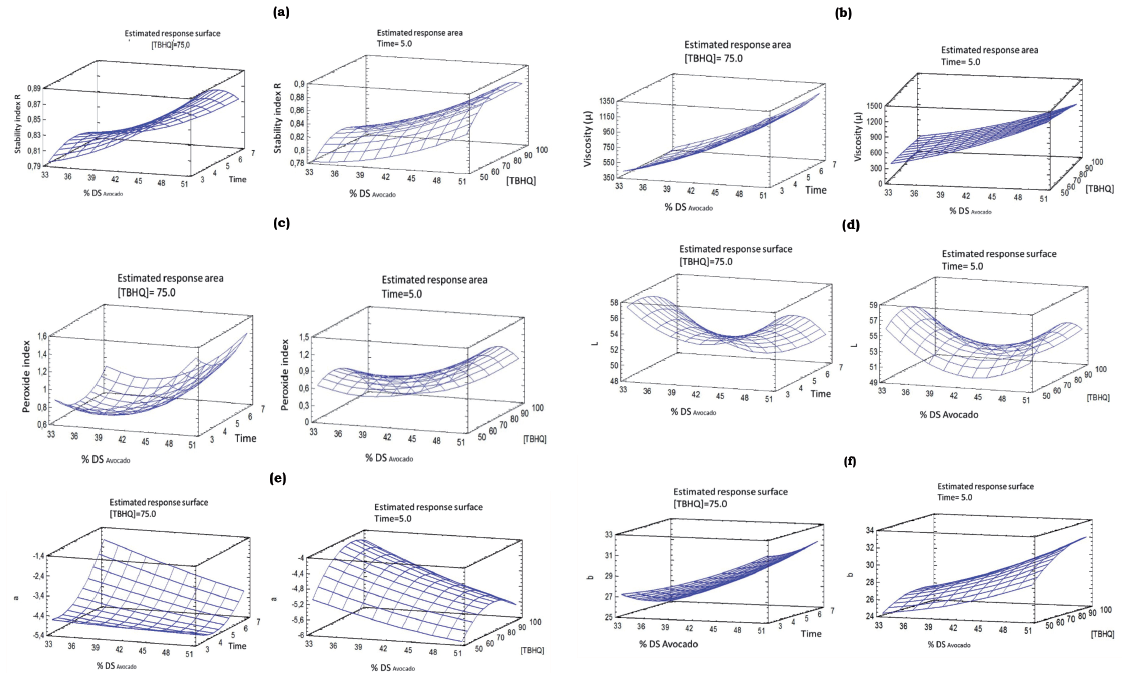
Figure 1 Response surface graphs of the quality attributes for emulsions based on avocado (Persea americana Mill. cv. Hass) and other ingredients depending on the evaluated independent variables
Viscosity (µ)
The DSAvocado (A) and [TBHQ] (C) lineal effects was significantly (p <0.05) in viscosity (µ), which fluctuated between 338.66 and 1395.33cP. In Figure1b, the increase of DSAvocado in formulation, increases the emulsion viscosity, due to the higher ratio DSAvocado/water. However, to higher DSAvocado, is where the effect of [TBHQ], becomes more important tending to reduce viscosity (µ) with the [TBHQ] increased. The emulsion viscosity decreased with increasing spindle speed, probably with shear rate increased. The drop-droplet interaction is modified and eventually interrupted, which reduces floc size and decreases viscosity. Given these concerns, the prepared emulsions behave as a non-Newtonian pseudoplastic fluid (Mirhosseini et al., 2009). This behavior is usual in food emulsions, for example, in seasoning salad emulsions (Chatsisvili, Amvrosiadis, & Kiosseoglou, 2012), in orange juice emulsions (Mirhosseini et al., 2009), in gum arabic drinks (Dłuzewska et al., 2006), among others.
Peroxide index (PI)
As can be seen in Table 3, the linear effects of DSAvocado (A) and [TBHQ] (C), was significantly (p<0.05) in PI, as well as the quadratic effect of [TBHQ] (C2) (p<0.05). Figure 1c, shows that the increase in DSAvocado, contributes to increasing PI, attributable to the higher content of mono and polyunsaturated avocado fatty acids, which are susceptible to oxidative reactions mainly on the particles surface (Kishk & Elsheshetawy, 2013). This situation is similar to that reported by Topuz et al. (2014), in a sauce to marinate fish containing olive oil and pomegranate juice, for Kishk & Elsheshetawy (2013), in mayonnaise and by Paraskevopoulou et al. (2007), in seasonings for greek salads.
The C2 quadratic effect influence is evident in the curvature of Figure 1c, where the lower values of PI or PI increase delay are reached when the [TBHQ] Is of the order of 100 mg.kg-1, throughout variation range of the DSAvocado. TBHQ addition in concentrations of 100 mg.kg-1, reached its minimum value of 0.206 H2O2meq. Kg-1 emulsion when the DSAvocado were minimal (33%) and the hydrophobic antioxidant action at the fat-water interface, becomes more effective by reducing the oily phase oxidation (Kishk & Elsheshetawy, 2013).
Color
ANOVA performed significant differences (p<0.05) in L* and a* with respect to homogenization time (B), varying between (47.9 & 57.6) and (-2.8 & -6.3), respectively. Whereas, the b* chromaticity was significantly influenced by the DSAvocado (A), varying between 23.2 and 33.3. Homogenization time (B), had an inverse effect with L *, as this decreases with increasing homogenization time (Figure 1d), which could be attributable to a slight browning experienced by the avocado (Persea americana Mill. cv. Hass) based emulsions and other ingredients, which is enhanced with homogenization time, by increasing its temperature from 25 °C to 32° C. On the other hand, it is attributed the observed browning of enzymatic type, due to the polyphenolic content and the present polyphenol-oxidase, which is potentiated with the air occluded during the homogenization process, which also favors lipids oxidation with the hydroperoxides and free radicals production (Kuhn & Cunha, 2012), which induces the found color changes. This phenomenon, intensifies to a longer homogenization period.
The a* chromaticity performed a decrease with the homogenization time (Figure 1e), which could be attributable to the greenish chromaticity decrease as a consequence of chlorophyll pigments and carotenoids degradation present in avocado (Aguiló-Aguayo, Oms-Oliu, Martín-Belloso, & Soliva-Fortuny, 2014), which is also potentiated at higher temperatures and greater air occluded in the emulsion. The b*chromaticity (yellow-blue) was significantly (p <0.05) influenced by the DSAvocado linear effect, increasing with the increase of DSAvocado (Figure 1f), indicating an increase in yellow chromaticity.
Particle size
Particle size reported as D10, D50 and D90 percentiles was not influenced (p> 0.05) by any of the considered independent variables. In general, particle sizes were large and fluctuating D10 (2.337- 22.324 µm), D50 (25.007 - 509.622 µm) and D90 (90.244 - 1110.778 µm), which was not considered critical, because it was sought when the reconstituted powder could be perceived similar characteristics to traditional guacamole, with pieces of vegetables of various sizes. Figure 2, presents particle size distribution of the emulsions at the optimum point, observing a trimodal behavior denoting their variability.

Figure 2 Particle sizes distribution of the emulsion under optimum conditions of composition and homogenization process
At this point, the mean values from 3 replicates correspond to D10= 8.1 µm, D50= 56.2 µm and D90= 346.6 µm. This trimodal or bimodal behavior, has been frequent in emulsions of oil-in-water type, for example, in seasoning salad emulsions, a proportion of relatively large particles is present, probably due to the aggregates formation during course of preparation , Which does not appear to diminish with homogenization (Chatsisvili et al., 2012). A bimodal behavior was presented in a sauce (milk emulsion) (Marco-Molés, Llorca, H. & Pérez-Munuera, 2012) and emulsions with linseed oil (Kuhn & Cunha, 2012).
Adjustment of response surface models
Table 4, presents the estimated regression coefficients of the polynomial response surface models with the corresponding R2 values and the non-fit test. Although, R2 values for the response variables were not high enough, the lack of fit, which measures the model fitness, resulted in no significant p-value (p> 0.05) in terms of the evaluated response variables, which indicates models which were adequate to describe the data behavior.
Table 4 Regression coefficients, R2 and probability values of the non-fit models for statistically significant variables

Optimization
In order to obtain experimental optimization using statistical tolos for more suitable values of the independent variables, which have allowed to obtain a final product with desired quality attributes. For this case, the following considerations were considered as criteria for optimization: an approximate viscosity of 1000 cP (suitable operating condition of the pilot dryer for avocado (Persea americana Mill. cv. Hass) based emulsions) with the highest percentage of DSAvocado, an emulsion which retains its luminosity (> L *) and greenish chromaticity (<a *), minimizing enzymatic browning and lipids oxidation (<IP), which were significantly influenced by homogenization time. Finally, a concentration of TBHQ capable of delaying or reducing the PI, which represents a critical variable in the process. The optimal conditions for the composition homogenization and process, which were defined as 47.1% of DSAvocado, since it corresponds to the highest percentage of DSAvocado, with a permissible viscosity to perform the aspersion-drying processes, 5 min for the homogenization time as the average level scanned where the color attributes are preserved and 100 mg.kg-1 for the TBHQ concentration, where the minimum values are reached for the Peroxide index. At this point, the experimental values obtained from three replicates to the optimal experimental conditions were potential-ζ -27.67 ± 0.29, the color parameters L * 51.3 ± 1.0, at * -5.8 ± 0.8 and b * 30.0 ± 1.9, viscosity 1034.56 ± 95.91 cP, spectral absorption stability index (R) 0.78 ± 0.03, peroxide index 0.73 ± 0.30 H2O2meq.kg-1 emulsion and particle size D10 8.1 ± 0.7 μm, D50 56.2 ± 11.5 μm and D90 346.6 ± 94.6 μm.
Conclusion
The results indicates this methodology with a central composite design is an effective technique to model the dependent variables as a function of the independent variables. In addition, have allowed to define the most appropriate independent variables to obtain a stable emulsion from the physical and chemical point of view. The obtained results from the emulsion based on avocado (Persea americana Mill. cv. Hass) and other ingredients, indicates an influenced stability for the formulation and homogenization operation conditions. The increase in DSAvocado Increased stability in terms of viscosity (µ) and PI, although had an inverse influence on R. On the other hand, the increase of homogenization time, affects the browning of the colloidal system. Whereas, the increase in [TBHQ] had a PI control, which could be associated with the lipid peroxidation inhibition.

















