Introduction
The Andean dry forests are ecosystems with high flora and fauna diversity (Madsen & Balslev, 2001). Lack of interest in these ecosystems have allowed a critical situation, an increasing in the risk of extinction due to the pressure of human activities (Janzen, 1988). This habitat is present as patches or fragments in almost all Neotropical region. Most of the time, they are surrounded by crops and areas dedicated to livestock (Fajardo, Gonzalez, Nassar, Lacabana, Portillo, Carrasquel & Rodríguez, 2005). In Ecuador, 35% (28000 Km2) of the Ecuadorian western region was covered by dry forest (Sierra, 1999). In fact, Sierra (1999), estimated that 50% of the dry forests have disappeared. An alternative for the flora and fauna regeneration and preservation of the dry forest, is agroforestry. This branch of agriculture is partially responding to deforestation problems, ecosystems degradation and the sustainability of livestock and agriculture (Amezquita, 2002).
Vachellia macracantha (Humb. & Bonpl. ex Willd.) Seigler & Ebinger, is a predominant species of trees of the inter-Andean dry forests due to its high capability to tolerate low periods of rainfall. V. macracantha belongs to the Fabaceae family and Mimosoidea subfamily. An important trait of this species is root nodule formation, which implies the presence of Ensifer or Rhizobium rhizobacteria, which are highly effective in fixing nitrogen (Cordero, Ruiz, de la Peña, Balaguer, Lucas, Rincón & Pueyo, 2016). The flowers of these trees are important for recruiting different pollinators, predominantly bees (Raine, Pierson & Stone, 2007). Additionally, the canopy hosts numerous insects that draw migratory birds, making V. macrantha a viable option for conservation. Due to nitrogen fixation and soil organic matter supplying, in addition to shade providing and windbreak, V. macrantha becomes an ideal species to associate with grazing lands for livestock (Greenberg, Bichier & Sterling, 1997).
Vegetative propagation is a common method to generate plant clones, however, implies using plant growth regulators, which involves more expenses. On the other hand, deforestation reduces species diversity and erodes the genetic basis for adaptation and survival. In order to increase genetic diversity within a species, is necessary to promote plant breeding through sexual crossing (seed production) (Olatunji, Maku & Odumefun, 2013). Sexual propagation of V. macracantha is limited due to the low percentage of seed germination. The seeds are covered by a hard surface layer, which prevents access of water and oxygen to the embryo, a state known as dormancy (Rodriguez, Eguiatre & Hernandes, 1982; Baptista & Quintão, 2017).
Seed dormancy is the inability of an unscathed viable seed to complete germination under propitious conditions. There are two types of seed dormancy: exogenous and endogenous. Seeds with exogenous dormancy usually have pericarp and/or seed coat impermeable to oxygen and/or water. Endogenous dormancy occurs when germination is inhibited by chemicals in epidermis or adjacent interior membranes (Bewley, 1997).
To overcome seed dormancy and to trigger seed germination, different techniques can be applied depending the dormancy type. Gibberellic acid and stratification, reduce endogenous dormancy by leaching out inhibitors. While scarification (physical or chemical treatments), are used to break down exogenous dormancy by removing or permeabilizing the seed coat (Matilla, 2008).
In order to create alternatives that favor propagation of V. macracantha that will help to conserve the Andean dry forests, is necessary to determine the most efficient seed scarification method for massively multiplying this species. The aim of this study was to evaluated seed germination of V. macracantha using chemical and physical scarification methods. Leaf number and plant height was also evaluated as a parameter of plant fitness.
Material and methods
Seed collection
In this study, V. macracantha seeds were obtained from an adult tree (Figure 1) located in Puembo at Tumbaco valley, close to Quito, Ecuador (Latitude: S 0° 20' / S 0° 10' Longitude: W 78° 30' / W 78° 15). Seeds were extracted from pods using mechanical methods. Each pod contains 15 seeds approximately. All seeds that were damaged, burned, pierced or had irregular shapes, were removed. Before any scarification treatment, the seeds were soaked in distilled water for 24 hours.
Chemical scarification treatment
Three acids were used for chemical scarification: sulfuric acid (H2SO4), nitric acid (HNO3) and phosphoric acid (H3PO4) at 50% v/v (Hernandez, Tizad, Freites, Diaz, Torrealba & Rodríguez, 2011). Treatments of five different immersion (soaking) times were tested as follows: 5, 10, 15, 20 and 25 minutes, respectively. After immersion, all the seed were washed with distillated water three times. Each treatment was identified by two capital letters: SA (sulfuric acid), NA (nitric acid) and PA (phosphoric acid), followed by a number indicating the number of minutes that seeds were immersed.
Physical scarification treatment
Two mechanical scarification and one thermal scarification methods were tested. For mechanical scarification, seed coats were filed with a metal file or nicked with pliers. Thermal scarification involved imbibing the seeds in water at 96°C for 10 minutes (Rodriguez, Eguiatre & Hernandes, 1982). Each treatment was identified by two capital letters: FI (filed), NI (nicked) and TS (thermal scarification).
Statistical analysis
A total of 19 treatments were tested, and 90 seeds per treatment were evaluated. Three replicates were carried out with an interval of three weeks. Two months after planting, three variables were measured as follows: germination percentage, number of leaves and plant height. Germination percentage was calculated as number of germinated seeds over total seeds planted. Number of leaves was the total sum of leaves per plant. Plant height was measured in centimeters using a tape measure. Randomized block design was applied, and the data were analyzed using analysis of variance ANOVA to identify significant difference among treatments and statistical significance for all comparisons was made at p<0.05. Tukey's multiple range test was used to compare the mean values of treatments.
Results
Figure 2, shows the results for the three variables (percentage of germination, number of leaves and plant height) for all chemical treatments. There are no significant differences among treatments, included control in all the evaluated parameters. These results showed that chemical scarification methods do not improve the germination of V. macracantha seeds.
Germination test
All seeds obtained through scarification method and the control (no scarification) treatment, were planted in plastic seedbeds trays, each tray had 200 square cells (1.5 x 1.5 cm), each cell containing 12 mL of substrate. A commercial peat moss substrate (Stender AGTM, Co., Germany), was used. The substrate contained 85% of blond peat and 15% mix peat, pH 5.5 to 6.0 and 0.9 g.L-1 of mineral content NPK (14% N, 16% P2O5, and 18% of K2O, respectively). All trays were placed in a greenhouse under the same conditions (Temperature ranging from 15 to 28°C with 75% of relative humidity). The seeds were watered every three days for two months, when data were collected.
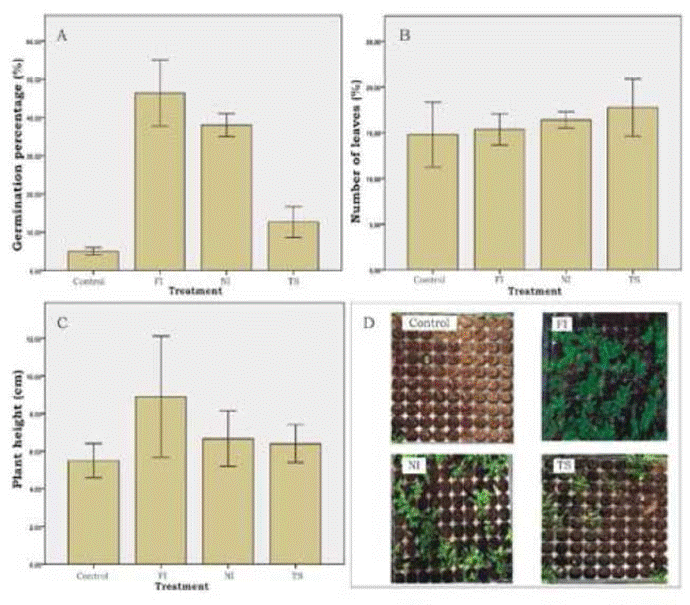
Each treatment is labeled by two capital letters: FI (filed), NI (nicked) and TS (thermal scarification). Three variables are shown: (A) germination percentage, (B) number of leaves and (C) plant height. In panel D, a seedbed tray at two months after sowing is shown for each treatment, pointing out differences in seed germi nation. *Significant differences were found using ANOVA at 5% significance level. Bars represent standard deviation.
Each treatment is labeled by two capital letters: NA (nitric acid), PA (phosphoric acid) and SA (sulfuric acid), followed by a number indicating the number of minutes that seeds were soaked. Three variables are shown: (A) germination percentage, (B) number of leaves and (C) plant height. No significant differences were found using ANOVA at 5% of significance level. Bars represent standard deviation.
All physical scarification treatments, produced a significant increasing in seed germination percentage when compared to control treatment (Figure 3). Filing and soaking treatments had achieved the higher seed germination percentages: 46.3% and 38%, respectively, compared to the control (6%) (Figure 3A). Thermal scarification also produced an increasing in seed germination percentage (12.7%). Number of leaves (Figure 3B) and plant height (Figure 3C), do not present significant differences for any evaluated treatment.
Discussion
Chemical scarification
All acids used failed to improve the seed germination percentage at any soaking time. This can be attributed to the concentration since at 50% (v/v), acids tested at this concentration might not turn permeable the seed coat, which have allowed no entrance of water to the seed embryo. In fact, Seed coats may act as physical barriers limiting water uptake, gas exchange and/ or embryo expansion (Huang, Mayton, Amirkhani, Wang & Taylor, 2017). Low germination levels of dormant seed are related to tissues surrounding the embryo (Tian, Knapp, Moore, Brummer & Bailey, 2002; Baptista & Quintão, 2017; López, López, Hernández, Charrez, González, Muñoz & Ortiz, 2017).
Hernández, Tizad, Freites, Diaz, Torrealba & Rodríguez (2011), had achieved 95% of germi nation in Acacia macracantha (black cuji), using concentrated sulfuric acid (96% v/v) during 12 minutes. Comparing these results could be inferred that high concentration of sulfuric acid is necessary to scarified V. macracantha seeds. In our study, we used half of the concentration tested by Hernandez et al. (2011), however exposure time was double than the mentioned research without any increasing in seed germination.
Another important parameter is immersion time. The periods used in this study, up to 25 min, were not long enough to produce signifi cant differences. In another study in Lupinus bilineatus,Martinez, Rodríguez, Guizar & Bonilla (2008), used 50% (v/v) sulfuric acid between 30 to 40 minutes getting 82% of seed germination. Even though sulfuric acid was used at the same concentration, the immersion time applied by Martinez, Rodríguez, Guizar & Bonilla (2008), was almost the double than the time used in the present study.
However, other species would need higher acid concentration with longer immersion periods. In a study carried out by Sanabria, Silva, Oliveros & Barrios (2001), in Centrosema rotundifolium, 96% sulfuric acid for 32 minutes were necessary to produce a significant improvement in germi nation percentage when compared to the control. Based on these findings, is necessary to try longer immersion times (higher than 30 minutes) and an increasing acids concentration (more than 50% v/v) to expect higher seed germination percentages in V. macracantha.
Physical scarification
Seed germination percentage were significantly improved through all physical treatments compared to the control. Mechanical treatments break down the integrity of the seed coat, which facilitates water absorption and embryo expansion (Huang et al., 2017).
These results are comparable in variability to the report by Rodriguez, Eguiatre & Hernandes (1982), in a thermal scarification treatment of Leucaena spp., seeds of this species produced higher germination percentage. The treatment consisted of embedded the seeds in boiling water for 3 minutes. Although the seeds were embed ded into hot water for 10 min, only 12% of seed germination percentage were obtained. Time of thermal scarification must be adapted to each species with the aim to obtain an increasing germination percentage without affecting seed quality and fitness.
Physical scarification methods showed to be more effective than chemical scarification me thods in this study. In other study carried out by D'Aubeterre & Gercia (2002), chemical scarification was most effective than physical methods to improve the germination of two from three species of the Prosopis genus.
Conclusion
The results obtained in this study are useful to agroforestry and propagation of V. macracantha, which provides more accurate and reliable estimates of the evaluated scarification methods. Bearing this in mind, the use of physical scarification method, have allowed an increasing seed germination from 6% (control) to 46.33% (filed). In addition, these methods do not produce any effect on the number of leaves and plant height respect to plant fitness. Given these concerns, these methods facilitate a more precise propagation of V. macracantha to promote reforestation of this species in dry forests areas.