Introduction
Sacha inchi (Plukenetia volubilis) belongs to family Euphorbiaceae, occurs in disturbed areas or forest edge of lowland moist or wet forest and is mostly found from sea level to less than 1000 m of altitude (Bussmann, Paniagua-Zambrana and Torres, 2013; Gillespie and Armbruster, 1997). It is widespread in the Lesser Antilles and South America, where it is found primarily in the northern and western regions and margins of the Amazon Basin in Surinam, Venezuela, Colombia, Ecuador, Peru, Bolivia and Brazil (Gillespie and Armbruster, 1997). Sacha inchi has been cultivated since pre-hispanic times in Peru and nowadays, it is a new oilseed crop incorporated into agricultural activity in the Amazon (Brack, 1999; Cachique, Rodriguez, Ruiz-Solsol, Valle- jos and Solis, 2011). The incorporation of little-known crops like sacha inchi into sustainable agricultural systems provides new developmental opportunities and can increase the resilience of food production systems (Cachique, Solsol, Sánchez, López and Kodahl, 2018). Furthermore, it has a high potential for replacing illicit crops (Cachique et al., 2011) and the establishment of commercial plantations generates positive environmental impacts because it can be installed on degraded soils (Solis, Cachique, Guerrero-Abad, Ruiz and Tapia, 2018).
In recent years, its importance in the international market has increased remarkably due to its seeds have high content of proteins and unsaturated fatty acids, mainly omega 3, 6 and 9 (Guillén, Ruiz, Cabo, Chirinos and Pascual, 2003; Gutiérrez, Rosada and Jiménez, 2011). However, its commercial exploitation is still incipient because no varieties have been developed, it is susceptible to root-knot nematode (Meloidogyne incógnita) -the main disease- and has a wide genetic, morphological and phytochemical variability (Marquez-Dávila, Gonzales, Arévalo and Solis, 2013; Rodríguez et al., 2010).
Sacha inchi is an allogamous species (Valente et al., 2017) and is usually propagated by seeds, therefore, its offspring is heterogeneous. Vegetative propagation allows maintaining 100% of genetic identity of superior genotypes, ensures the conservation of germplasm and increases the genetic gain in short periods by using both the additive and non-additive components of the genetic variance (Solis, Pezo, Díaz, Arévalo and Cachique, 2017; Zobel and Talbert, 1988). Furthermore, the propagation by rooting cuttings presents advantages such as conferring precocity, multiplying species that do not produce seed and multiplying more plants in reduced spaces (Cachique et al., 2011; Solis et al., 2017).
Lack of reliable methods for the asexual propagation has limited the establishment of commercial plantations because genotypes with outstanding traits cannot be massively propagated (Ruiz-Solsol and Mesén, 2010). Rooting cuttings has been a suitable form of vegetative propagation because of the success in obtaining homogeneous crops (Silva, Costa, Ferreira, Silva and Gomes, 2010). Previous studies have established rooting methodologies for sacha inchi and Plukenetia polyadenia, a wild relative of sacha inchi, using subirrigation propagators and sand as substrate (Cachique et al., 2011a Solis et al., 2017). The sand allows good aeration, good drainage and provides adequate support for the rooted cuttings (Cachique et al., 2011), but the cuttings must be transplanted to another substrate for acclimatization and the root system suffers damages during this process. The use of Jiffy pellets during the vegetative propagation in microtunnels allows to use the same substrate for rooting and acclimatization, avoiding the stress of rooted cuttings and reducing propagation costs (Badilla and Murillo, 2005). This substrate showed good results in rooting cuttings of Calycophyllum spruceanum (Vallejos-Torres, Toledo and Arévalo, 2014).
To achieve an adequate rooting cuttings in microtunnels it is necessary to establish a nursery with three main conditions: (1) a reduction in the photosynthetic activity with the use of shadow, (2) a high relative humidity (80 - 90 %) and adequate control of water stress, and (3) a temperature of approximately 30 °C (Badilla and Murillo, 2005).
Although cuttings offer a practical and easy method to obtain seedlings in different vegetable species (Bonilla, Sánchez and Perlaza, 2007; Dalla-Rosa et al., 2018), the limitations are the high financial cost for the construction of microtunnels and the necessity of trained personnel for handling and maintenance of the plants. Therefore, it is necessary to propagate large quantities of sacha inchi plants with superior genetic traits for reducing production costs and also for satisfying the demand of high genetic quality seedlings for the installation of commercial plantations.
In this context, this study aims at developing a method for rooting juvenile cuttings of sacha inchi using microtunnels in order to propagate plants with superior genetic traits and shorten production cycles.
Materials and methods
This study was conducted in the greenhouse of the Peruvian Amazon Research Institute, San Martin, Peru. The rooting of juvenile cuttings of sacha inchi was performed using microtunnels, adapting the methodology developed by Vallejos-Torres et al. (2014) for Calycophyllum spruceanum. In this process were used fresh basal cuttings of 8 cm, which were collected from mother plants with superior phenotypic characteristics (high yield, high levels of unsaturated fatty acids and tolerance to the root-knot nematode).
For the rooting process, a microtunnel of 3 m long, 1.5 m wide and 0.6 m high was used, with a structure formed by a half-inch welded iron frame, placed horizontally and arched to form the microtunnel. The structure was lined with transparent polyethylene plastic. The base had support of iron and metallic mesh for placing the trays that contained the substrates with the sacha inchi cuttings. Likewise, the microtunnel has three nebulizers for irrigation and drains to avoid the excess of water inside it (Figure 1) (Vallejos-Torres et al., 2014).

Figure 1 Microtunnels used for the rooting of sacha inchi cuttings. Instituto de Investigaciones de la Amazonia, San Martín, Perú.
Due to the high humidity generated inside the microtunnel, a microclimate with favorable conditions for the propagation of fungi and other pathogens was created, so it was necessary to remove the fallen leaves and cuttings with necrosis. The cleaning of the internal surface of the microtunnel was carried out once a week, with soap and water, in order to prevent the spread of pathogens.
During the first experiment two types of substrates (sand and Jiffy pellets) and two nebulized irrigation frequencies were tested (once per day and three times per day, each application was for 30 seconds). The Jiffy pellets were hydrated with plenty of water for 15 minutes, until they reached the maximum volume. The cuttings of eight cm were obtained from vigorous shoots collected in the early hours of the day. With the help of a micropipette, 10 pl of indolebutyric acid (IBA) solution at a concentration of 2000 ppm was applied at the base of the cuttings and then were placed on the substrate at two cm deep, according to the methodology developed by Cachique et al. (2011). The sand and the Jiffy pellets were placed on trays and the distance among the cuttings in the sand was 10 x 10 cm. Then, all cuttings were transferred to the microtunnels. A completely randomized block design was used with four treatments, three replications per treatment and 10 cuttings per experimental unit.
In the second experiment three levels of leaf area (25, 50 and 75 cm2) and five concentrations of IBA (0, 1000, 2000, 4000 and 6000 ppm) were tested. The substrate and irrigation frequency used were selected from the results obtained in the first experiment. A completely randomized block design with 15 treatments, three repetitions per treatment and 10 cuttings per experimental unit was used.
The evaluation was performed 12 days after placing cuttings in the substrates. Data were submitted to an analysis of variance and Tukey test (P < 0.05) to determine the nature of the differences among treatments. Prior to the analysis, the data percentages were transformed using arcsen and the count data were transformed by (Snedecor and Cochran, 1980). The statistical analysis was performed using the software SAS 9.4, released on 2013.
Results and discussions
The optimization of a methodology for the vegetative propagation of sacha inchi will allow producing clones with genetic and phytosanitary high quality for the establishment of commercial plantations and also, will be a useful tool in the process of breeding because it shortens the production cycles. The environmental conditions are influential in this process and in this sense, Leakey and Mesén (1991) reported that low temperatures within the propagator are important for two reasons: (1) the evaporation rates are lower, and (2) the water retention capacity of the air (humidity) is dependent on the temperature, therefore, low temperatures help avoid water stress by maintaining high relative humidity. Previous studies in vegetative propagation of sacha inchi and its wild relatives by rooting cuttings in sub-irrigation propagators showed that the optimum temperature within the propagation area that favors rooting is from 25 to 30 °C, with a relative humidity of approximately 90% (Ruiz-Solsol and Mesén 2010; Solis et al., 2017). The values registered in the microtunnels in this study are within the reported ranges and contributed to the success of rooting of sacha inchi cuttings.
Determination of the type of substrate and frequency of irrigation
The most important factors associated with the rooting of cuttings in a substrate are aeration, good drainage, easiness for sterilization and adequate support for the cuttings (Hartmann, Kester and Davis, 1997). In this study, the analysis of the Tukey test indicated that the treatment which involve the use of sand and the frequency of three daily irrigations and the treatment which involve the use of Jiffy pellets and the frequency of a daily irrigation showed the highest percentages of rooting and the lower percentages of mortality, statistically superior from the rest of treatments. Moreover, the use of Jiffy pellets and frequency of daily irrigation favored the development of a greater number of roots with an adequate average length, which would benefit the process of acclimatization (Table 1).
Table 1 Effects of the type of substrate and frequency of irrigation on rooting cuttings of sacha inchi in microtunnels. Instituto de Investigaciones de la Amazonia, San Martín, Perú.
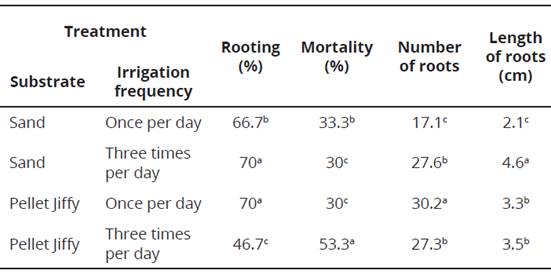
*Means followed by equal letters in the columns do not differ according to Tukey test (P < 0.05).
The vegetative propagation of sacha inchi cuttings requires a special substrate that can be used during the rooting and acclimation phases. The sand allows to obtain good results in rooting cuttings (Cachique et al., 2011) but then, they must be transplanted to another substrate for acclimatization and the root systems suffer damages in the process. However, the use of the Jiffy pellets allows to use the same substrate for rooting and acclimatization, avoiding the stress of rooted cuttings and reducing the costs of propagation (Badilla and Murillo, 2005), and showed good results in the direct rooting of cuttings of Calycophyllum spruceanum (Vallejos-Torres et al., 2014).
The irrigation system in the microtunnels for rooting cuttings was nebulized and automatic. The use of sand required an irrigation frequency of three times per day, with a duration of 30 seconds each one. The treatment in which was used sand and a daily irrigation did not allow the adequate supply of water for the cuttings and consequently were obtained low percentages of rooting (Table 1). Badilla and Murillo (2005) suggested increasing the frequency of irrigation on sunny days and reducing it on rainy days. They also reported that if the rooting of cuttings is done using pellets, the irrigation frequency should decrease to once per day for 30 seconds. In this study the use of Jiffy pellets and a daily irrigation allowed obtaining high percentages of rooting in comparison with the treatment of three daily irrigations. The cuttings inside the Jiffy pellets that were irrigated three times per day suffered a water saturation affecting the emergence and development of roots and consequently, causing high mortality. Therefore, appropriate moisture in the rooting substrate is essential to obtain satisfactory results (Fachinello, Hoffmann, Nachtigal, Kersten and Fortes, 1995). In a complementary way, the physiological conditions of the mother plants, especially the level of auxins, were influential in the process of rooting (Oliveira, Pasqual, Chalfun, Regina and Rincón, 2003).
Determination of the leaf area and IBA concentration
The objective of the second experiment was to determine the leaf area of the cuttings and the concentration of IBA suitable for rooting. According to the results obtained in the first experiment, Jiffy pellets and a daily irrigation for 30 seconds were selected for use in this second experiment.
The cuttings must conserve part of the leaf because they are source of photo assimilates and auxins for the rooting. However, the leaf also provides a large surface for water loss by transpiration. For these reasons, the leaves should be cut in a size that achieves the best balance between the disadvantages of transpiration and the advantages of photosynthesis (Mesén, 1998). The adequate leaf area of the cuttings varies for each species and in the case of sacha inchi, good results were obtained with areas of 50 and 100 cm2 (Cachique et al., 2011). In this study, the use of 75 cm2 allowed obtaining high percentages of rooting and good root development. However, although several studies indicate that the leaf area is directly proportional to the number and length of roots, in this study was observed that the root development was more linked to the IBA concentration than to the leaf area (Table 2).
Table 2 Effects of the leaf area and IBA concentration on rooting cuttings of sacha inchi in microtunnels. Instituto de Investigaciones de la Amazonia, San Martín, Perú.

*Means followed by equal letters in the columns do not differ according to Tukey test (P < 0.05).
The purpose of treating cuttings with IBA is to increase the percentage of rooting, reduce the time of root initiation and improve the quality of the root system formed. The IBA is an effective auxin to promote the rooting cuttings in a large number of species (Hartmann et al., 1997) and showed positive responses in sacha inchi (Cachique et al., 2011). The cuttings that were not treated with IBA showed low percentages of rooting, so it is deduced that the endogenous auxins influence in the root development in vegetative propagation processes of sacha inchi. Concentrations of 2000, 4000 and 6000 ppm allowed to obtain high percentages of rooting (Table 2) but 4000 and 6000 ppm induced the development of callus at the base of the cuttings and can inhibit the growth of roots and shoots, like was observed in Eplingiella fruticosa (Silva, Oliveira and Silva, 2017). Cachique et al. (2011) mentions that concentrations higher than 2000 ppm are toxic for sacha inchi cuttings and decrease the percentage of rooting. Furthermore, Vallejos-Torres et al., (2014) observed that the percentage of rooting in cuttings of Calycophyllum spruceanum decreased with the increase of IBA concentrations. Therefore, according to the results the appropriate concentration for rooting sacha inchi cuttings in microtunnels is 2000 ppm (Figure 2). All the treatments presented low mortality percentages except the treatment with 25 cm2 of leaf area and 1000 ppm of IBA. The different response of this treatment is probably due to mechanical manipulation processes.

Figure 2 Cuttings of sacha inchi with 75 cm2 of leaf area rooted in microtunnels with Jiffy pellets and 2000 ppm of IBA, 12 days after the treatment. Instituto de Investigaciones de la Amazonia, San Martín, Perú.
The number and length of roots showed an increasing tendency with the increase of IBA concentration, as it has been observed in previous rooting studies of sacha inchi cuttings (Ruiz-Solsol and Mesén, 2010). Root formation was influenced by the capacity of cuttings to provide carbohydrates to areas where the roots emerge. However, it is important to note that concentrations higher than 2000 ppm induced the development of callus at the base of the cuttings, affecting the normal development of plants in the processes of acclimatization and adaptation in the field.
Rodrigues, Bordignon and Ambrosano (2014) evaluated in the field the horticultura! performance of sacha inchi plants propagated by in vitro techniques and showed higher yield in comparison to plants propagated by seeds. Therefore, it is necessary to carry out this type of studies with plants propagated by rooting of cuttings in microtunnels, and evaluate the yield and morphological development, in order to compare plants vegetatively propagated in microtunnels with the conventional plants propagated by seeds. In addition, vegetative propagation through rooting cuttings in microtunnels will be validated as a tool to support the genetic improvement of sacha inchi.
Conclusión
The use of microtunnels for sacha inchi propagation allows having a greater control of the internal environmental conditions, irrigation frequencies and the phytosanitary status of the cuttings. In addition, it is possible to use the same substrate in the processes of rooting and acclimatization, avoiding damages in the roots. The use of cuttings of 8 cm with 75 cm2 of leaf area, 2000 ppm of IBA, Jiffy pellet as substrate and one nebulized irrigation per day induced high percentages of rooting (93.3%) and the best root formation. This method can be used in breeding programs because it allows multiplying massively outstanding genotypes and shortens production cycles of sacha inchi.














