Introduction
Myelomeningocele (MMC) is the most common and severe form of spina bifida, and it affects 1-10 out of every 1000 live births in the world. This congenital defect occurs at approximately 4 weeks of gestation and is characterized by incomplete neural tube closure. This results in nerve fibers being in direct contact with amniotic fluid, rubbing constantly against the walls of the uterus.1-3
Patients with MMC have multiple complications, including hydrocephalus, motor impairment of the lower limbs, and bladder and gut dysfunction; orthopedic and neurocognitive abnormalities and abnormalities related to the Chiari type II malformation; and emotional and social changes.2,4
With the boom of fetal surgery, intrauterine MMC repair emerged as an option to limit damage and complications associated with it.5 MOMS (Management of Mielomeningocele Study) was first put into motion in 2003 with the aim of comparing outcomes between intrauterine repair and conventional postnatal repair over an 8-year period. Results showed that prenatal surgery reduced the need for cerebrospinal fluid shunting, led to improved motor function, and reduced hindbrain herniation. However, it was also associated with a higher risk of preterm delivery and uterine dehiscence during cesarean section.6
Advancements in fetal surgery create new challenges for anesthetists, whose role is critical for the success of the surgery. This case report is the first successful experience in intrauterine MMC repair in Peru.
Case presentation
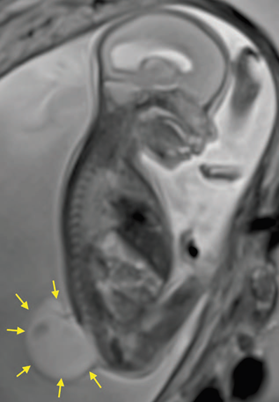
A 27-year-old primigravida at 25 weeks of gestation with fetal diagnosis of open spina bifida (MMC), ventriculomegaly, and Arnold Chiari II syndrome, scheduled for intrauterine MMC correction. Figure 1 shows the magnetic resonance image revealing the extensive fetal vertebral defect. The patient had no relevant personal history and the physical examination found American Society of Anesthesiologists (ASA) Physical Status classification II, weight 84kg, height 169 cm, body mass index 29, blood pressure (BP) 110/70 mm Hg, heart rate 74/min, absent difficult airway predictors, and no abnormal cardiopulmonary findings. Laboratory tests included hemoglobin 12.3 g/dL, prothrombin time (PT) 11.5 seconds, partial thromboplastin time (PTT) 26.2 seconds, fibrinogen 310mg/dL, and platelet count 234,000/µL. The patient and her spouse were explained the anesthetic techniques with their risks and benefits and it was decided to use an epidural catheter for postoperative pain management, and general intravenous anesthesia, and the informed consent was obtained.
In the operating room, a thermal blanket was used, a peripheral No. 18 line was placed in the left arm and the patient was administered ranitidine 50mg, metoclopramide 10 mg, and cefazolin 2g. A second intravenous No. 16 line was placed immediately in the same arm. Basic ASA monitoring was applied, and difficult airway devices and aspiration catheter were prepared. The patient was then placed in a left lateral decubitus position, asepsis and antisepsis were performed, and the epidural space was identified 5 cm from the skin surface using a No. 18 Tuohy needle and the technique of intermittent loss of resistance, at the level of L2-L3. The No. 20 multiport epidural catheter was then put in place. A test dose was given using 3 mL of 2% lidocaine with epinephrine 1 in 200,000, with a negative result.
The patient was then positioned in supine decubitus and an infusion with remifentanil 1.5 ng/ml concentration in the effect site (Ce) was initiated using target control infusion (TCI). After asepsis and antisepsis and local anesthetic infiltration, an arterial catheter was placed in the right arterial artery. Following preoxygenation, rapid sequence induction was initiated with remifentanil 5 ng/ mL Ce by TCI, propofol 4 µg/mL Ce by TCI and rocuronium 1 mg/kg. The Sellick maneuver was performed, achieving intubation on the first attempt, and with no significant changes in hemodynamic parameters. The remifentanil and propofol infusion was set to reach a bi-spectral index between 40 and 60, and a fluctuation in hemodynamic parameters of less than 20% in relation to initial values. In turn, the remifentanil infusion was used to achieve transplacental fetal anesthesia. Surgery was initiated and at the time of uterine exposure to access the fetus, a nitroglycerine infusion was used ranging between 1 µg/ kg/minute and a maximum of 6 µg/kg/minute to favor uterine relaxation and allow neurorrhaphy by the neurosurgeon, as illustrated in Fig. 2. Ethylephrine infusion was used at a dose of 2.5-5 µg/kg/minute to maintain systolic BP > 90 mm Hg and mean arterial pressure >60mm Hg, and a crystalloid infusion was administered at a rate of 20 mL/hour. The nitroglycerine infusion was interrupted at the end of the hysterorrhaphy, and a bolus dose of atosiban 6.75 mg was administered followed by a 300 µg/ minute infusion. At the end of surgery, 0.125% bupivacaine plus fentanyl 50 µg were administered through the epidural catheter in a total volume of 16 cm3. Analgesia was supplemented with the administration of metamizole 2-g intravenous and the patient was then extubated following reversal with neostigmine and atropine, with full recovery from total neuromuscular blockade.
The surgery was uneventful. Postoperative analgesia was managed with a continuous peridural infusion of bupivacaine and fentanyl, plus tocolytic drugs during the first 48hours. The patient remained in the intensive care unit during the first 3 postoperative days with intermittent pneumatic compression to prevent thromboembolic events, and was then transferred to the ward where she remained until discharge on postoperative day 7. No postoperative complications were reported. Laboratory test results at discharge were hemoglobin 12g/dL, PT 11 seconds, PTT 26 seconds, fibrinogen 387mg/dL, platelet count 230,000/µL
The patient was readmitted at 37 weeks of gestation for elective cesarean section using spinal-epidural technique, with no intraoperative or postoperative complications, and was delivered of a newborn adequate for gestational age, weighing 2879 g, with 1 and 5 minute Apgar scores of 8 and 9, respectively. Figure 3 shows the neonate with completely healed spine. Assessment of the newborn by the pediatric neurologist at 24hours found no hydroceph-alus or Arnold Chiari II malformation, and discreet lower limb motion.
Discussion
The exact incidence of neural tube defects (NTD) in our country is unknown. Tarqui-Mamani et al described an overall in-hospital NTD incidence rate of 13.6 for every 10,000 live births between 2001 and 2005 at the National Maternal Perinatal Institute in, Lima, Peru, and the most frequent form of spina bifida was MMC with an overall incidence rate of 7.4 for every 10,000 live births.7 Following the national intervention of wheat flour fortification with folic acid, the overall incidence rate of NTD and MMC dropped to 8.73 and 6.0 for every 10,000 live births, respectively, in the period between 2006 and 2010.8
In the MOMS study, apart from the benefits of prenatal repair for the neonate, it was found that there was increased maternal morbidity including the risk of hysterorrhaphy dehiscence, uterine rupture secondary to hysterotomy, and the need for cesarean section in all subsequent pregnancies.9 For this reason, risk/benefit must be assessed on an individual basis, taking into account the considerations from all the specialties involved as well as patient-related factors.
A key point is the need for uterine relaxation to create optimal conditions for surgery and to maintain adequate uteroplacental perfusion, and it is worth noting that neuroaxial anesthesia does not provide uterine relaxation.
In normal conditions, the uterine muscle contracts in response to the stimulus. This myometrial contractility is inhibited by calcium-sensitive potassium channels modulated by volatile anesthetics.10 Many authors use and recommend the use of high-dose volatile anesthetics (minimum alveolar concentration > 2) to maintain uterine relaxation.11 Potential disadvantages of this technique are hemodynamic instability and excess uterine bleeding. Moreover, high-dose inhaled agents have been associated with significant fetal cardiac dysfunction and a risk of neuronal apoptosis described in animal studies,12 whereas a study by Chatterjee et al13 showed that the use of propofol with desflurane minimized fetal cardiac dysfunction.
Another option to maintain uterine relaxation is the use of a nitroglycerine infusion. Nitroglycerine induces smooth muscle relaxation and produces generalized vasodilation.14 In obstetrics, it has been widely used in 50 to 100 µg boluses in cases of placental retention. However, its administration increases the incidence of maternal pulmonary edema during these types of surgeries, probably because it acts as a nitric oxide donor, creating nitrite peroxide which harms type II pneumocytes.9 This constitutes an additional challenge in terms of monitoring and restricted fluid administration. Despite these considerations, we selected the latter option given the quick reversibility of the effect and independence from the depth of anesthesia.
Any drug used to maintain uterine relaxation will give rise to significant maternal hypotension given uteroplacental perfusion dependency and inability to autoregulate, hence the importance of using vasopressors.
Different vasopressors are used in obstetric anesthesia, phenylephrine being the drug of choice due to its lower repercussions on fetal pH as compared with ephedrine. Belzarena15 compared ephedrine and ethylephrine and found no significant difference between their use and neonatal or maternal outcomes in patients under spinal anesthesia for cesarean section. In their study on the use of ethylephrine and phenylephrine in spinal anesthesia, Bolanos et al16 found them to be equally effective in the treatment of hypotension associated with spinal anesthesia for cesarean section, with no differences in fetal outcomes. Based on these results, and given the unavailability of phenylephrine in the country, we decided to use ethylephrine infusion.
Finally, fetal anesthesia during this procedures is provided by transplacental crossing of drugs such as volatile anesthetics and opioids, among others.9 In vitro studies have shown that inhaled anesthetics (sevoflurane) produce a dose-dependent contractile depression of the myocardium, which may be reduced when used together with intravenous agents.17 We selected the transplacental route with the use of remifentanil.
The main strengths of this study include coordinated team work and the availability of equipment, monitors, and supplies for this type of surgery. The main limitation of this report is the description of anesthetic management in a single case.
Conclusion
Each and every detail of the anesthetic management will contribute to the success or failure of this type of surgery. It is clear to us that the understanding of maternal and fetal physiology is the starting point for continuing with this type of procedure, avoiding potential maternal complications and improving fetal prognosis. The use of total intravenous anesthesia associated with nitroglycerine infusion for uterine relaxation, ethylephrine infusion to maintain maternal hemodynamic stability, and adequate postoperative pain management with epidural analgesia was successful in this case.
Ethical responsibilities
Human and animal protection. The authors declare that no experiments were conducted in humans or animals for this research.
Data confidentiality. The authors declare having followed the protocols of their institution regarding disclosure of patient information.
Right to privacy and informed consent. The authors obtained the informed consent from the patient and/or subjects mentioned in this article. The informed consent form is available from the corresponding author.











 text in
text in