What do we know about this problem?
Respiratory viral infection outbreaks exhibit varying levels of bacterial and fungal coinfections. Influenza-related infections account for up to 30% of community-acquired pneumonia cases. COVID-19 patients have a 7-14% chance of bacterial coinfection, while superinfections can lead to a higher risk of mortality and longer hospital stays. Multidrug-resistant bacteria are also a concern due to the widespread use of antibiotics and deficient infection control and prevention measures.
What does this study contribute?
This study contributes to understanding the behavior of healthcare-associated infections (HAI) in severe COVID19 patients admitted to the intensive care unit (ICU) in Colombia. The prevalence of bloodstream infections (BSI) was 35.2%, while the prevalence of coinfections was 7.69%, which is lower than reported in other studies. The study found a significant association between ICU-acquired infection and having diabetes mellitus and the Acute Physiology and Chronic Health Evaluation (APACHE) II score. Gram-negative bacteria were the most frequently isolated germs, and Klebsiella species bacteria were the most commonly isolated. The study highlights the high frequency of carbapenem-resistant Klebsiella pneumoniae, which is a cause for concern.
INTRODUCTION
Respiratory viral infection outbreaks have shown variable behavior in terms of bacterial and fungal coinfections; for example, it has been reported that influenza-related infections can account for up to 30% of community-acquired pneumonia cases, mainly in older adults. 1 In addition, a systematic review that included studies on clinical outcomes in the 2009 H1N1 pandemic reported that coinfection was detected in 23% of cases. 2 In the case of other coronaviruses, coinfections have only been addressed in a few studies conducted in patients with SARS-CoV 3 and MERS-CoV, 4 where the frequency of coinfection has been reported to be 41% and 0%, respectively.
Regarding the prevalence of coinfections in patients infected with SARS-CoV-2, a meta-analysis that included 30 studies (3834 patients) reported that 7% of hospitalized COVID-19 patients had a bacterial coinfection, and 14% in those admitted to the ICU. 5 The bacterial pathogens detected were different from those described in patients with influenza, as no cases of coinfection with Streptococcus pneumoniae or Streptococcus pyogenes were found. There was only one case of methicillin-resistant Staphylococcus aureus, and Mycoplasma pneumoniae, pseudomonas aeruginosa, Haemophilus influenzae and Klebsiella pneumoniae were the most frequently isolated bacteria. 5
On the other hand, superinfections affecting patients with COVID-19, especially those requiring ICU admission, are at the other end of the spectrum. Bloodstream infection (BSI) has been described in up to 50% of COVID-19 patients within 30 days of ICU stay, 6 almost double the prevalence of BSI observed in other cohorts of ICU patients. 7 Similarly, patients with COVID-19 have a higher risk of developing ventilator-associated pneumonia which could be attributable to the severity of lung damage and the use of immunomodulators. 8 Finally, in COVID-19 patients admitted to the ICU, bacterial and fungal infections are associated with a longer hospital stay and a higher risk of mortality. 9 Another concern is the high prevalence of multidrug-resistant (MDR) bacteria due to the widespread use of broad-spectrum antibiotics, the aberrant immune response and the subsequent failure of this immune response, 10-12 the failure of standard ICU care measures, as well as deficiencies in the availability of personal protective equipment and the reduction of infection control and prevention measures in this clinical setting. 13
Given the need to establish the local behavior of infections in patients with COVID-19 admitted to the ICU, the intent was to describe the pathogens isolated in blood and tracheal secretion cultures obtained from COVID-19 patients admitted to the ICU, identifying antibiotic resistance patterns, and to evaluate the association between the presence of secondary infections and 60-day mortality.
METHODOLOGY
A retrospective cohort analytical study was conducted, including adult patients admitted to the ICU of a quaternary referral hospital in Bogotá D.C., with severe COVID-19 (confirmed diagnosis by means of SARS-CoV-2 real-time polymerase chain reaction test (N=322)), between April 15 and December 31, 2020.
Participants were recruited using convenience sampling. Patients in whom blood cultures were not performed during their stay at the ICU, those who had been more than 72 hours at the ICU of another hospital, those in which a poor life expectancy was determined based on their clinical condition, and those who died within the first 24 hours after admission were excluded; hence, the final sample comprised 273 patients.
The study was conducted at the Subred Integrada de Servicios de Salud del Sur - Hospital El Tunal, a hospital that offers quaternary care services and has become a reference center in Bogotá D.C. for the management of patients with severe COVID-19, upon expanding its ICU capacity during the pandemic (3 ICUs vs. 9 ICUs, with a maximum of 123 ICU beds available for this purpose).
Procedures
Compliance with all the mandatory laboratory and imaging tests according to the institutional ICU care protocol within the first 72 hours after ICU admission was verified for all patients, based on the review of their medical records. The following information was also collected from their medical records: demographic data, clinical manifestations of the disease, history of disease, laboratory and imaging test results, SOFA, APACHE II and CURB-65 scores on admission to the ICU, results from blood tracheal secretion cultures, length of ICU stay, length of hospital stay, and the development of complications, including death, during hospital stay and 60 days after ICU admission.
All the blood and tracheal secretion culture isolates were reviewed by an infectious disease specialist, who established whether microbial colonization was present and determined the antimicrobial resistance patterns based on the results of antibiograms or antifungal susceptibility testing. Furthermore, the cause of the infection was established by consensus between the infectious disease specialist and the researchers of the study.
Operational definitions: Coinfection was defined as the existence of a secondary infection considered to be concurrent with the initial diagnosis of COVID-19 based on the isolation of any germ in a blood or tracheal secretion culture performed within 48 hours after ICU admission. 14 All infections were defined according to the criteria of the Centers for Disease Control and Prevention (CDC). 15 Any cases of bacteremia due to coagulase-negative Staphylococci isolated from a single blood culture were considered as contaminated blood cultures. 16
Statistical analysis
Initially, a descriptive analysis was performed by calculating means and standard deviations (SD) for continuous variables and absolute and relative frequencies for categorical variables. Additionally, bivariate analyses were performed to determine possible differences between groups (positive blood cultures and negative blood cultures) using the Chi-square and the Student's t-tests for categorical and continuous variables, respectively.
Kaplan-Meier curves were constructed to estimate survival according to the presence of positive blood cultures, candidemia, infections caused by Klebsiella species, carbapenem resistance and beta-lactamase-producing bacteria (BLEE and AMP-C). A statistical significance level of p<0.05 was established using the log-rank test. Furthermore, a bivariate analysis was performed using a Cox proportional hazards regression model to evaluate the association between 60-day mortality and the presence of positive blood cultures, candidemia or carbapenem-resistant germs by calculating hazard ratios (HR) with their respective 95% confidence intervals (95%CI). Finally, a multiple logistic regression analysis was carried out to assess the association between the presence of coinfections and a number of clinical and laboratory variables, which were identified in the literature review as related to this outcome: presence of type 2 diabetes mellitus, APACHE score, SOFA score, CURB-65 severity score, C-reactive protein (CRP) level, lymphocyte count, leukocyte count, lactate dehydrogenase levels and ferritin levels.
Although the sample size was based on convenience sampling, the Freeman rule was followed for constructing the multivariate regression model considering our exploratory analytic approach. This rule suggests having at least 10 outcome events for each variable included. All the analyses were done using the R statistical software version 4.0.2 (R Foundation, Vienna, Austria), with the "survival", "pROC" and "ROCit" packages.
RESULTS
Of the 273 patients, 95 (34.8%) were women and the average age was 60.4 (SD= 14.2) years. Table 1 shows the main characteristics of the population; high blood pressure (39.9%), type 2 diabetes mellitus (21.2%) and chronic lung disease (19.8%) were the most frequent comorbidities. The average length of hospital stay was 23.5 (SD= 15.2) days and 144 (52.7%) patients died within 60 days after admission. There were no significant differences between patients with a positive blood culture versus a negative blood culture result in any of the variables analyzed (Table 1). It should also be noted that all patients received antibiotic treatment in the ICU and that 93.5% received corticosteroids.
Table 1 Demographic, clinical and laboratory characteristics of the sample.
| Variable | Total sample (n= 273) | Patients with a positive blood culture result (n= 96) | Patients with a negative blood culture result (n= 177) |
|---|---|---|---|
| Females, n (%) | 95 (34.8%) | 33 (34.4%) | 62 (35.0%) |
| Age in years (mean; SD) | 60.4 (14.2) | 61.5 (12.7) | 59.9 (15.0) |
| Comorbidities, n (%) | |||
| High blood pressure | 109 (39.9%) | 36 (37.5%) | 73 (41.2%) |
| Type 2 diabetes mellitus | 58 (21.2%) | 27 (28.1%) | 31 (17.5%) |
| Chronic heart disease (except for high blood pressure) | 34 (12.4%) | 13 (13.5%) | 21 (11.9%) |
| Chronic kidney disease | 11 (4.0%) | 5 (5.2%) | 6 (3.4%) |
| Smoking | 67 (24.5%) | 17 (17.7%) | 50 (28.2%) |
| Chronic pulmonary disease | 54 (19.8%) | 20 (20.8%) | 34 (19.2%) |
| Chronic neurological disorder | 11 (4.0%) | 3 (3.1%) | 8 (4.5%) |
| Cirrhosis | 4 (1.5%) | 2 (2.1%) | 2 (1.1%) |
| Use of immunosuppressants | 8 (2.9%) | 2 (2.1%) | 6 (3.4%) |
| Duration of COVID-19 symptoms before ICU admission (in days) (mean; SD) | 8.4 (4.3) | 7.9 (4.2) | 8.7 (4.4) |
| Laboratory tests | |||
| White blood cell count (x103 cells/µL) (mean; SD) | 13.0 (12.5) | 13.2 (10.4) | 12.9 (13.6) |
| Lymphocyte count (x103 cells/µL) (mean; SD) | 1.0 (1.2) | 1.1 (1.3) | 1.0 (1.1) |
| Lymphocyte count <of 1.2 X103 cells/µL, n (%) | 217 (79.5%) | 70 (72.1%) | 147 (83.0%) |
| Platelet count (x103 cell/µL) (mean; SD) | 238.6 (95.6) | 234.1 (98.1) | 241.0 (94.4) |
| PaO2/FiO2 ratio (mean; SD) | 101 (63) | 97.8 (66.4) | 103 (62) |
| Lactate level (mmol/L) (mean; SD) | 2.1 (2.0) | 2.2 (2.5) | 2.0 (1.8) |
| Creatinine level (mg/dL) (mean; SD) | 2.4 (4.1) | 3.0 (4.6) | 2.1 (3.7) |
| Aspartate aminotransferase level (U/L) (mean; SD) | 111.2 (339.1) | 159.8 (549.1) | 86.3 (137.4) |
| Alanine aminotransferase level (U/L) (mean; SD) | 98.2 (216.1) | 99.0 (218.6) | 97.9 (215.6) |
| C-reactive protein level (hs-CRP test) (mg/L) (mean; SD) | 18.1 (14.8) | 18.0 (13.3) | 18.0 (15.5) |
| Ferritin level (ng/mL) (mean; SD) | 1161.2 (640.9) | 1191.2 (659.5) | 1146.0 (632.9) |
| D-dimer level (µg/mL) (mean; SD) | 41.2 (459.7) | 4.9 (7.2) | 60.0 (565.9) |
| Lactate dehydrogenase levels (U/L) (mean; SD) | 1476.8 (7603.8) | 1010.9 (5055.9) | 1725.8 (9411.9) |
| Positive high-sensitivity cardiac troponin I (cTnI) test, n/ n tested (%) | 109/259 (42.1%) | 44/92 (47.8%) | 65/167 (38.9%) |
| Clinical prediction rules | |||
| SOFA (Sequential Organ Failure Assessment) score (mean; SD) | 4.8 (3.3) | 5.0 (3.3) | 4.7(3.3) |
| APACHE II score (mean; SD) | 12.9 (6.5) | 13.5 (6.5) | 12.6 (6.5) |
| CURB-65 score (mean; SD) | 1.9 (1.1) | 1.9 (1.2) | 1.9 (1.1) |
| Outcome | |||
| Length of hospital stay (in days) (mean; SD) | 23.5 (15.2) | 25.7 (16.4) | 22.3 (14.5) |
| Death, n (%) | 144 (52.7%) | 53 (55.2%) | 91 (51.4%) |
APACHE II: Acute Physiology and Chronic Health Evaluation; SD: standard deviation; SOFA: Sequential Organ Failure Assessment Score. CURB-65: Confusion, Blood urea nitrogen, Respiratory rate, Systolic BP and Age > or = 65.
Source: Authors.
A total of 511 blood cultures were performed among the patients included. The mean time between ICU admission and the first blood culture was 4 days (SD=7.8). There were 40 (7.8%) contaminated blood cultures and at least one positive blood culture was reported in 96 patients (35.2%). On the other hand, at least one tracheal secretion culture was performed in 169 patients (61.9%), while in 62 (22.7%), the number of tracheal secretion cultures was 2.
Table 2 shows the distribution of the germs isolated in the blood and tracheal secretion cultures. In the case of blood cultures, of the 127 isolated germs 78 (61.4%) were gram-negative bacteria, 32 (25.2%) Candida and 17 (13.4%) gram-positive bacteria. K. pneumoniae was the most frequently isolated germ (34 isolates; 26.8%) followed by Candida albicans (16 isolates; 12.6%). Finally, of the 306 germs isolated in both blood cultures and tracheal secretion cultures, 121 (39.5%) were MDR germs, with carbapenem resistance being the most common antimicrobial resistance pattern (33.3% of gram-negative bacterial isolates).
Table 2 Distribution of germs isolated in blood and tracheal secretion cultures, and antimicrobial resistance mechanisms.
AMP-C: AMP-C-type becta-lactamase-producing bacteria; Carba-R: carbapenem resistance; ESBL: extended-spectrum beta-lactamase-producing bacteria.
Source: Authors.
In terms of timing of blood and tracheal secretion cultures, 214 blood cultures (41.9%) were performed within 48 hours after admission to the ICU, of which 5 (2.3%) were positive (two for S. aureus, one for E. coli, one for Enterococcus faecalis and one for C. albicans) and 15 were considered to be contaminated. On the other hand, 58 tracheal secretion cultures were performed within 48 hours after admission to the ICU, of which 16 (27.6%) were positive (7 cases of C. albicans, 5 cases of Klebsiella species, 2 of S. aureus, 1 of P. aeruginosa and 1 of Serratia marcecens). Coinfection was confirmed in 21 patients (7.69%).
Figure 1 shows the Kaplan-Meier curves to estimate 60-day survival probability after admission to the ICU according to the presence of positive blood cultures, candidemia, Klebsiella species infection, carbapenem resistance and beta-lactamase-producing germs (ESBL and AMP-C) without differences among the various groups.
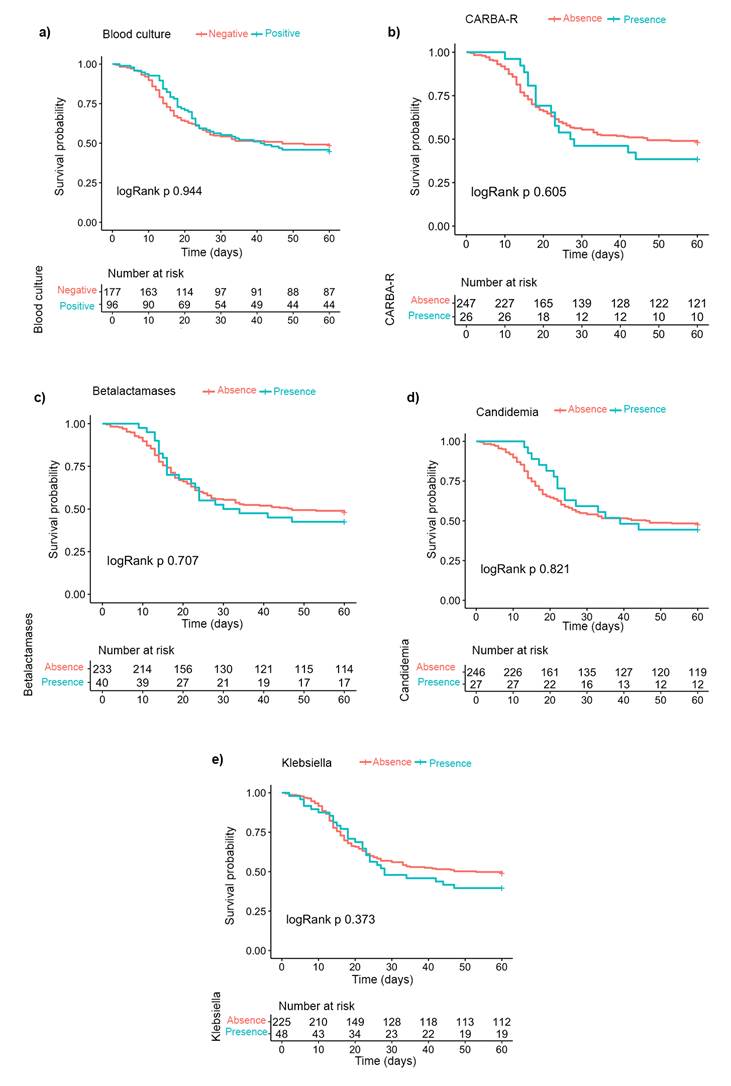
Source: Authors.
Figure 1 Kaplan-Meier estimation curves for survival at 60 days according to a) presence of positive blood cultures, b) presence of carbapenem resistance, c) presence of beta-lactamase-producing germs (ESBL and AMP-C), d) presence of candidemia and e) presence of Klebsiella species in blood cultures.
The following HRs for the association between 60-day mortality and the presence of positive blood cultures, candidemia and carbapenem-resistant germs were obtained based on the Cox proportional hazards regression model: 1.012 (95%CI= 0.721-1.420; p=0.946), 0.939 (95%CI= 0.550-1.603; p=0.818) and 1.148 (95%CI= 0.682-1.931; p=0.604), respectively. Finally, the results of the multiple logistic regression analysis are shown in Table 3, where the presence of type 2 diabetes mellitus and the APACHE II score were associated with the presence of coinfection (OR: 1.086; 95%CI=1.005-1.172; p=0.033 and OR: 1.010; 95%CI=1.005-1.015; p<0.001 respectively).
Table 3 Multiple logistic regression analysis of the association between several variables considered and the presence of coinfections.
| Variable | OR | 95%CI | p value | |
|---|---|---|---|---|
| Type 2 diabetes mellitus | 1.086 | 1.005 | 1.172 | 0.033 |
| APACHE score | 1.010 | 1.005 | 1.015 | <0.001 |
| SOFA score | 1.007 | 0.997 | 1.016 | 0.172 |
| CURB-65 score | 1.022 | 0.994 | 1.050 | 0.123 |
| PCR level | 0.999 | 0.997 | 1.002 | 0.636 |
| Lymphocyte count | 1.000 | 1.000 | 1.000 | 0.854 |
| Leukocyte count | 1.000 | 1.000 | 1.000 | 0.682 |
| Lactate dehydrogenase levels | 1.000 | 1.000 | 1.000 | 0.829 |
| Ferritin levels | 1.000 | 1.000 | 1.000 | 0.819 |
Source: Authors.
DISCUSSION
Given the overwhelming number of patients with severe COVID-19 that have often used up the ICU capacity in Colombia during the COVID-19 pandemic, it is essential to describe the behavior of health care-associated infections. In this regard, the prevalence of BSI in this study was 35.2%, a figure similar to the pooled prevalence of 29.6% (558/2487 patients) reported by Ippolito et al. 7 in a meta-analysis of 46 studies (42694 patients) and the 39.74% described by Giacobbe et al. 6 in 78 critically ill Italian patients.
On the other hand, the prevalence of coinfections in patients with COVID-19 admitted to the ICU was 7.69% (21/273 patients), which is lower than the 14% reported in the meta-analysis by Lansbury et al.5 However, this difference could be attributed to several causes, such as the fact that in this study there is a possible bias derived from the non-systematic performance of blood and/or tracheal secretion cultures on admission to the ICU (only 41.9% of blood cultures were performed within 48 hours after admission), the unavailability of rapid diagnostic tests for various germs such as antigen or multiplex polymerase chain reaction (PCR-multiplex) testing, and the widespread exposure to broad-spectrum antibiotics at the time the cultures were performed, which undoubtedly has an impact on its microbiological performance when conventional techniques are used.
Another relevant finding in our study was the significant association between the presence of ICU-acquired infection and having diabetes mellitus and the APACHE II score; this is consistent with what has been reported by Bardi et al. 9 in 140 patients, showing that the development of these infections was significantly associated with APACHE II score, having diabetes and use of corticosteroids.
Regarding the type of germs isolated in both blood and tracheal secretion cultures (n=306), gram-negative bacteria were predominant (61.4%), followed by fungi (25.2%), and only 13.4% were gram-positive bacteria; this differs from the findings of Bardi et al. 9, who reported that of the 91 episodes of ICU-acquired infection identified, 55% were caused by gram-positive bacteria, 30% by gram-negative bacteria and 15% by fungi; however, regarding the prevalence of MDR germs, our results (39.5%) are similar (33%). Likewise, in this study, Klebsiella species bacteria were the most frequently isolated germ (35.6%), while other bacteria such as E. coli, P. aeruginosa, S. aureus and Stenotrophomonas maltophilia were significantly less frequent (9.45%, 3.15%, 3.98% and 0.79%, respectively), a situation that, although similar regarding the types of bacteria that were isolated, differs in terms of frequency from the bacterial distribution behavior described by Chong et al. 18 It should be noted however, that these differences may be due to the inherent characteristics of the local microbial flora in each ICU.
K. pneumoniae accounted for 35.6% of isolated gram-negative bacteria and, of these, 40.5% were carbapenem-resistant (i.e. 14.4% of all isolated germs). In this regard, several authors have highlighted the high frequency of Klebsiella pneumoniae carbapenemase (KPC) producing bacteria, such as Mędrzycka-Dąbrowska et al,13) who reported that the prevalence of KPCs ranged from 0.35 to 53% over 11 studies. Similarly, a systematic review that included 38 articles reported that, of the 1959 isolates, 29% were MDR strains,19 being methicillin-resistant Staphylococcus aureus, carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Klebsiella pneumoniae and carbapenem-resistant Pseudomonas aeruginosa the most frequent. Since 2018, the World Health Organization has prioritized the control of carbapenem-resistant germs.20 In this regard, several studies have shown a possible increase in the frequency of isolated germs with this mechanism of antibiotic resistance during the COVID-19 pandemic; for example, a study conducted in patients who developed at least one nosocomial infection reported that the rates of resistance to imipenem and meropenem were higher in COVID-19 patients 21. This situation may be challenging when choosing the first-line antibiotic therapy; hence, the promt administration of PCR tests for the detection of these germs (e.g. film array) could have an impact on the prognosis of these patients.
In patients with infections caused by carbapenem-producing enterobacteriaceae (CPE), Pintado et al. 22 reported that mortality in patients with COVID-19 was high (43.3%) and that 61.5% of these deaths were mainly caused by CPE. Similarly, Polly et al.23, in a study conducted in Brazil with data from 2641 healthcare-associated infections caused by MDR bacteria, reported a worrying, although not significant increase in the incidence of CPE between the pre-pandemic period and 2020 (1.29 vs 1.38; p=0.42). The above evidence highlights the need to improve both hospital-acquired infections surveillance, prevention and control measures and antimicrobial use optimization programs, which failed in several countries during the pandemic due to the increased frequency of MDR germs, particularly carbapenem-resistant gram-negative bacteria, as well as the need to implement strategies to control these microorganisms as an essential component of the hospital management of patients with COVID-19.24
Pasero et al. (14 in a recent systematic review including 12 studies on secondary bacterial infections in patients with COVID-19 admitted to the ICU, report that invasive mechanical ventilation was the only risk factor associated with the presence of MDR microorganisms (OR: 1.062, 95%CI: 1.012-1.114), although they also point out that the use of steroids and extended ICU stay could also play a key role; furthermore, MDR germs are associated with a longer ICU stay, but not with an increased risk of death. 13 In our study, the presence of secondary infections was not significantly associated with 60-day mortality (HR: 1.012, p=0.946), which is also similar to what is reported in the study conducted by Ramanan et al. 25 where the association between mortality and the presence of bloodstream infections was not significant (OR: 1.61, p=0.3). However, in the study by Ferrando et al.26 despite a higher proportion of respiratory superinfection and BSI in patients who died, (33 vs. 25%; p=0.03 and 33 vs. 23%; p=0.01, respectively), this association was not consistent in the multivariate analysis. However, other studies have reported a significant association between mortality and the presence of coinfection: Silva et al.27 report that the risk of death was higher in patients with positive cultures for bacteria (OR: 11.28) and fungi (OR: 5.97); Baskaran et al.28 describe that patients with coinfection were more likely to die in the ICU, and Bardi et al.9 found a significant association between mortality and the development of a nosocomial infection.
Finally, it should be noted that this study has several limitations, including the non-systematic performance of blood cultures on ICU admission, the unavailability of rapid diagnostic tests, and the unavailability of genomic tests to determine the presence of antibiotic resistance mechanisms. Moreover, its retrospective design could lead to identification and selection bias. Notwithstanding the above, and despite the fact this is not a multicenter study, the data herein reported are representative of a broad population group, since the hospital where the study was conducted is a referral center in the southern area of Bogotá D.C., servicing nearly 2 million people, in addition to the significant expansion of its ICU capacity. Moreover, this is so far the first study of its kind conducted in this area of influence, representing a significant contribution in this field of study, not only in Bogotá D.C., but also in the country. We acknowledge however that the small sample size and the potential presence of confounding factors and bias may have influenced our findings, limiting our ability to detect significant differences in clinical outcomes and coinfections-associated mortality. These limitations underscore the need to be cautious when interpreting our conclusions and highlight the importance of conducting further studies with larger samples and more robust analyses to control for these factors.
CONCLUSIONS
Although the prevalence of superinfection was moderately high in this study, the prevalence of coinfection was low. Moreover, there was a predominance of gram-negative bacteria and almost one third of the isolated microorganisms were MDR, with carbapenem resistance being the most frequent antibiotic resistance pattern.
ETHICAL DISCLOSURES
Ethics committee approval
The study protocol was approved by the Institutional Ethics Committee of the Subred Integrada de Servicios de Salud del Sur - Hospital El Tunal. Considering the health crisis caused by the COVID-19 pandemic, the Committee decided that the submission of a signed informed consent was not necessary, as provided under Minutes No. 138 of 2020. Likewise, the ethical principles for conducting biomedical research involving human subjects established by the Declaration of Helsinki were followed.
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics commit-tee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).











 text in
text in 



