INTRODUCTION
Spinal anesthesia (SA) in children is long-established. As with many innovations, the popularity of SA has fluctuated. By the mid-twentieth century, the usage of SA had decreased, primarily due to the improved safety of general anesthesia (GA), the introduction of newer induction agents and an increase in number of professional anesthesiologists. A renewed interest in SA for pediatric surgery emerged in the late I970s and early I980s due to improved survival rates of extremely premature infants. Given the higher incidence of postoperative apneas after GA in this high-risk population, awake SA was shown to be a safer alternative 1. Our review will focus on SA for neonates, infants, and children. The term children will refer to patients 2 years of age and older.
ANATOMY
Understanding age-specific changes of critical anatomical structures is vital prior to administering pediatric SA. The fetal conus medullaris (CM) ascends as gestation progresses. However, when the CM in infants reaches the adult level Li, continues to be debatable 2. During the past decade, two research groups have reported slightly different levels of CM termination in newborn term infants depending on technology used. MRI studies report the lowest level as the inferior border of the L2 vertebra 3. If ultrasound (US) is used however, the lowest normal reported level is L3 2.
Tuffier's line remains a useful landmark as it passes the L4-5 interspace level in neonates or is shifted to the upper third of L5 vertebra in the flexed position 4,5. The dural sac in neonates and infants also terminates more caudally than in adults, at about S4 at birth and reaches S2 by the end of the first year, in contrast with the adult level of Si 1.
Historically, it was largely accepted that neonates have a significantly higher CSF volume. In the last decade newer in vivo MRI-imaging techniques found CSF volume in neonates and infants to be much lower at around 2 ml/kg without drastic decreases with increasing age 6,7. The clinical implication of this finding is to reinforce dosing IT administered local anesthetics (LA) according to weight because the volume in which they will be diluted is a direct function of weight 6. The findings cast doubt on the theory that higher CSF volume in neonates and infants might be a factor in shorter duration of SA 6,7. Other mechanisms such as higher CSF turnover, delayed myelination and pharmacological differences may be at play 6.
ULTRASOUND-GUIDED SPINAL ANESTHESIA
The use of US to assist in the administration of pediatric SA has been reported since the early 2000s 8,9. Initial reports focused on use of US to calculate the skin to subarachnoid space distance and assessing the feasibility of identifying critical neuraxial structures such as spinous process, dura, and CM. In the last decade, the focus has been on using preprocedural scans to objectively confirm CM termination level and identifying corresponding interspinous space (ISS) levels or continuous real time scan for delivering SA in previously difficult lumbar puncture (LP). The recent review of US-guided SA in infants concluded that there is no high-quality evidence to suggest that US use for SA is superior to the standard approach in the pediatric population 10.
An advantage of using US for neuraxial blocks in neonates and infants is the ease with which a good bone window can be discerned given its superficial location 10. This allows for consistent appreciation of the ISS and spinal cord meninges. In 2023 Du et al. showed that anatomical landmarking of ISS levels was inaccurately marked in 32% of infants planned for SA. In one patient, the intended ISS overlaid the CM, with the risk of experiencing spinal cord trauma 11.
Two probe orientations for visualizing the dura mater can be used: longitudinal (along the spine) or transverse (across the spine) (Figures 1 and 2). The longitudinal approach helps find the widest ISS and the level where the CM terminates by counting from the last rib downwards and from the lumbosacral junction upwards (Figure 3).
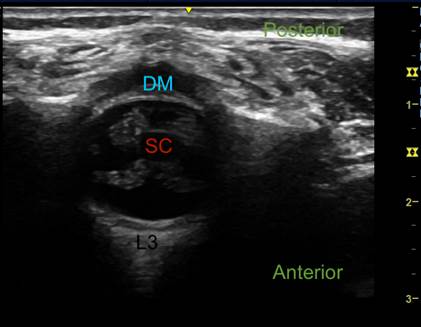
CSF: cerebrospinal fluid; DM: dura mater; SC: spinal cord. Source: Authors.
Figure 1 Ultrasound imaging of the vertebral canal. Transverse axis at level L3.
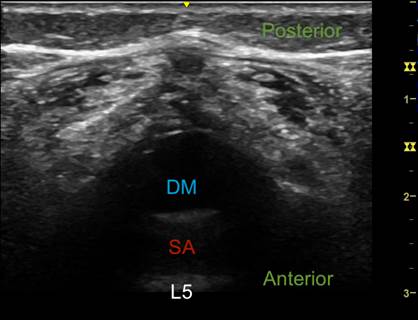
CSF: cerebrospinal fluid; DM: dura mater; SA: subarachnoid space. Source: Authors.
Figure 2 Ultrasound imaging of the vertebral canal. Transverse axis at level L5.
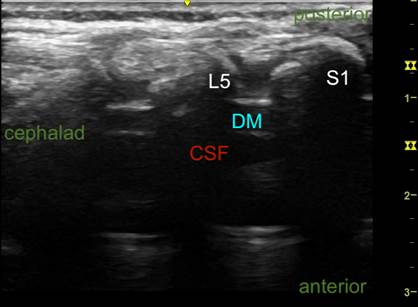
DM: dura mater; CSF: cerebrospinal fluid. Source: Authors.
Figure 3 Ultrasound imaging of the vertebral canal. Longitudinal axis.
A barrier to implementing US for pediatric SA is the challenge of simultaneously scanning and tracking the needle on awake and moving neonates and infants. The concern is that the process may take much longer than SA using anatomical landmarks. The skill level of the practitioner is an important factor in the success of US-guided SA. A retrospective report in 2019 of 14 ex-premature infants who underwent continuous US-guided SA for inguinal herniorrhaphy had a first pass success rate of 64% and 3-attempt success rate of 86% 12. The overall success rates are lower than those reported for landmark-based approaches of 98.9% 12.
POSITIONING
Proper positioning is crucial for a successful LP. Since SA is performed through LP, we added to our analysis publications based on LP to obtain more reliable data. Traditionally, in neonates and infants either the lateral decubitus or sitting position is used. A recent Cochrane review from 2023 suggests little or no difference in first-attempt success or time to perform an LP in either position 13. The incidence of bradycardia and oxygen desaturation is likely increased in the lateral decubitus position. There was little or no difference in the number of episodes of apnea in either position 13. The incidence of bradycardia and oxygen desaturation may be attributed to significant cervical flexion which increases the risk of airway obstruction. Optimizing positioning while limiting cervical flexion to avoid significant airway obstruction is encouraged. Many adolescents and teenagers require sedation for LP, making the sitting approach challenging. In such cases, the lateral decubitus position may be preferred. The inability to maintain optimal positioning and follow commands with sedation can make successful completion of the procedure difficult.
TECHNIQUE
In neonates the L4-5 or L5-S1 interspace should be identified and a median approach with needle advancement is recommended to appreciate the feel of the tight ISS, narrow subarachnoid space, low CSF pressure, and poorly calcified laminae 5,11. The L3-4 interspace may be used in infants. A 25 G 25 mm Quincke needle with stylet is often used. The level of the block in infants can be assessed by observation of profound motor weakness in the legs and a lack of response to skin pinch at the level appropriate for the surgery 14. For older patients, a 25 G Whitacre needle with 20 G skin introducer is commonly used.
When performing SA on awake infants, there are additional strategies to optimize conditions. These include applying a topical LA over the injection site, administering a small dose of dexmedetomidine or fentanyl intranasally (IN) 15, and providing a warm and quiet operating room (OR). The attending surgeon should remain present in the OR ready for surgical prep, and the SA duration should be monitored with a timer, informing the surgeons at 30, 45 and 60 minutes. A pacifier dipped in sucrose may be used. A peripheral intravenous (IV) catheter ideally should be placed in the lower extremity following SA administration 16. Temperature control should be maintained at all stages of the procedure, warming the room and using transparent coverings while spinal injection is administered. Interestingly, reports suggest that using an active warming device might disturb awake neonates and infants due to noise and irritation of the upper body 15.
LOCAL ANESTHETIC PHARMACOKINETICS
LA pharmacokinetics differ across age groups and is relevant to dose, duration of action and toxicity.
Binding
Important age-dependent differences impact LA binding in plasma up to the first year of life 17,18. Amide LA primarily bind to alpha-1 acid glycoprotein (AAG). The AAG serum concentration is several times lower at birth, progressively increasing during the first 6-9 months of life to reach adult levels by the end of the first year 19. This is of importance since the toxicity of LA agents is related to the unbound plasma concentration rather than to the total plasma concentration 17. Tetracaine primarily binds albumin, however it metabolizes rapidly and protein binding is not clinically significant 19.
Absorption
In this last decade, Frawley et al. reported on pharmacokinetic studies of levobupivacaine use in infant SA 17,20,21. From their analysis, reported Cmax values for median total venous plasma concentration and median unbound venous plasma concentrations were well below levels tolerated by adults 17. In 4 of the patients, a repeat 1 mg/kg dose was required due to initial block failure. Cmax levels in these patients, though almost doubled, were still well below toxic levels. The median Tmax was 30 minutes for a single SA dose 17. For the four patients who received a second dose of 1 mg/kg, median Tmax was 45 minutes 17.
Distribution
Knowledge on the volume of distribution (Vd) of amide LA derives from pharmacokinetic studies with caudal anesthesia. Bupivacaine is considered to have the highest Vd in neonates followed by infants, children, and adults; however, comparative data following IV administration are not available 19. Ropivacaine has a smaller Vd than bupivacaine and is thought to have the smallest Vd in neonates, infants, followed by children. It approximates adult levels by age 4 years 19.
Metabolism and clearance
Immature cytochromes and conjugating mechanisms result in prolonged metabolism and elimination of amide LA 19. The clearance rate for bupivacaine and ropivacaine is low at birth, but it increases during the first year of life. Lower intrinsic clearance, along with decreased serum protein binding, are the two factors that may increase the risk of toxic reactions in infants (19.
Clinical implications
The joint American and European Societies of Regional Anesthesia guidelines for dosage of commonly used LA in pediatric regional anesthesia suggest levobupivacaine, ropivacaine, bupivacaine and tetracaine for IT injection (Table 1) 18. In 2017, Frawley et al. reported no sex related differences in the ED50 and ED95 dosing of levobupivacaine and ropivacaine SA 21. As such, no modification of dosing based on infant spinals was recommended, unlike in adults where sex related dosing differences are seen. Although bupivacaine in many countries remains the most popular LA for SA, the pharmacokinetic profile of levobupivacaine shows a better safety margin than bupivacaine in infants. Even after a repeated spinal dose (2 mg/ kg) it did not produce concentrations associated with increased risk of toxicity 17,21. An equivalent dose of bupivacaine is more potent than levobupivacaine and ropivacaine. Levobupivacaine and ropivacaine have similar potency ratios 22. The higher lipid solubility of bupivacaine allows for greater partitioning into the spinal cord, resulting in a larger motor block that intensifies with increasing dosage 22. Kokki and Hendolin reported no difference between isobaric and hyperbaric bupivacaine solutions for SA in children 1-7 years in terms of success rates, spread, and duration of sensory and motor block 23. However, the General Anesthesia vs Spinal (GAS) study estimated lower failure rates of SA with 0.5% isobaric bupivacaine (6.2%) than with hyperbaric 0.75% bupivacaine (28.6%) or 0.5% levobupivacaine (20%) 24. It should be noted that the described failures could be due to a smaller volume of LA used to achieve the intended dose 24.
LOCAL ANESTHETIC PHARMACODYNAMICS
Spinal anesthesia and central nervous system function
Injection of LA into the IT space resulting in somatic and autonomic blockade of the spinal nerve roots is the mechanism of action for SA 27. SA should not impact the level of consciousness and brainstem functions, unlike GA. This is not always consistent with the clinical findings of pediatric SA, some of whom fall asleep shortly after the procedure 28. One of the well-known indicators of the depth of GA is the absence of corneal reflex. Contrary to the spinal roots' mechanism of action of SA, a series from the last decade showed patients aged 2-13 months experienced a loss of corneal and eyelash reflexes after SA 29. After intrathecal injection, all patients fell asleep, and their cardiorespiratory status was unremarkable 29. In 2021, Whitaker et al. reported a prospective pilot study in which 12 infants who underwent awake SA had recorded encephalography (EEG) 28. EEG showed increased slow wave activity and decreased beta activity compared to the awake state, with the sleep spindles suggestive of a normal sleep 28. According to a study from 2023, EEG patterns seen during infant physiological sleep support sleep-related mechanisms of sedation during awake SA 30. The mechanism of this phenomenon is still unknown although it is suggested to be due to a deafferentation effect and not to a direct effect on the brain. Clinically, this may explain the decreased need for supplemental IV anesthetics for sedation after pediatric SA.
Spinal anesthesia and hemodynamics
BP changes to SA are more frequent in adults than in children. The reasons for hemodynamic tolerance of SA in infants are not clearly understood but immature sympathetic tone and smaller lower limb blood capacity are suggested as contributing factors 31-33.
The GAS study demonstrated that patients receiving SA had a lower incidence of hypotension (mean arterial pressure, MAP < 45 mmHg), shorter duration of hypotension, including prolonged hypotension (MAP <35 mmHg during three consecutive 5 minutes interval between measurements) and fewer interventions to treat hypotension 34. Weight at time of the surgery and low intraoperative temperature were risk factors for hypotension 34. The incidence of bradycardia under SA in the Vermont Infant Spinal Registry (VISR) was 1.6% (26 patients) 14.Three patients experienced bradycardia associated with the onset of high spinal block 14.
Near-infrared spectroscopy monitoring shows a neutral effect of SA on cerebral blood flow (CBF), particularly cerebral regional oxygen saturation (rSO2). Investigators did not find a clinically significant change of rSO2 despite decreasing MAP and HR 35,36. Patients in this study did not receive any premedication or sedation. Care should be taken when using premedication and/ or sedation as part of the SA technique as it could potentially further affect rSO2. Most anesthetic agents decrease both CBF and cerebral metabolic rate (CMR); however, with a concomitant SA, the decrease in MAP and HR may decrease CBF further to compromise rSO2 37.
Pediatric SA effectively mitigates the stress response when undergoing cardiac surgery with cardiopulmonary bypass 38. In a prospective randomized controlled study, Humphreys et al. measured plasma catecholamines and lactate concentrations and showed that continuous SA with indwelling catheter reduced their levels more effectively than high dose IV opioids alone 38. The hemodynamic profile in the spinal group did not differ from the control group with high-dose IV opioid anesthesia.
Spinal anesthesia and respiratory function in newborns and infants
Premature neonates and infants younger than 60 weeks experience a higher incidence of GA-associated postoperative apnea 39. SA has been used as an alternative to completely avoid GA and airway instrumentation. The reduction in perioperative respiratory complications as a result of utilizing SA has shown promising results 24,39,40. In the GAS study, awake SA in infants significantly reduced the incidence of early postoperative apneas and, more importantly, a significantly lower incidence of apnea interventions beyond simple stimulation 39. In contrast, GA significantly increased the incidence of apneic episodes and interventions to treat them. The highest incidence of apnea in the GA arm was seen in preterm infants at 6.1% 39. Further, all events of CPR, reintubation or positive pressure mechanical ventilation overnight to resolve early postoperative apnea occurred in the GA group 39. Ex-prematurity was the strongest risk factor for apnea. Early apnea was also a strong predictor of late apnea 39. Infants undergoing surgery with SA rarely need supplemental oxygen and were less likely to develop oxygen-desaturation postoperatively (1 vs. 4%) 39. Dohms et al. in their meta-analysis, showed SA was significantly better than GA for any episode of apnea. Incidence of apneic events in infants in the SA group was 9% compared to 20% in the GA group 40. The number of patients who required mechanical ventilation post-operatively was also several fold higher in the GA group (13% vs. 1.9%) 40. Results from the VISR favor SA over GA with desaturation occurring in only 0.6% cases of SA 14. Recent publications on the use of SA for pyloromyotomy show no episodes of apnea in the SA group versus 25% in the GA group 41.
Neonates and infants rarely develop signs of compromised ventilation with a high spinal block. In the GAS study only one infant needed bag-mask ventilation 39. Infants under SA did not show any signs of impaired breathing mechanics with spinal block level up to T4 33. The incidence of higher-than-needed spinal block in the VISR was 3.8% (10 patients) 14. Five patients subsequently required endotracheal intubation 14.
ADJUVANTS FOR SPINAL ANESTHESIA
Block duration shorter than 90 minutes is a limitation of SA, and can be as short as 60 minutes in neonates 42,43. No new LA agents have become available in the last decade to allow for longer duration of pediatric SA and the use of adjuvants to extend block duration has been reported; however, the evidence is limited 44.
Further concerns surrounding neurotoxicity of spinal administration of drugs remain unsolved 45. All IT drugs must be preservative-free.
Clonidine and dexmedetomidine
Neonates and infants usually tolerate IT administration of clonidine better than adults due to the immaturity of the sympathetic nervous system and a smaller peripheral blood volume 44. When added IT in neonates (1-2 mcg/ kg), clonidine prolongs analgesia 46. However, IT clonidine is also associated with a higher incidence of postoperative apneas and transient bradycardia in the neonatal population 45,46. The safety of pediatric IT dexmedetomidine is not clear. In 2020, a report from Fares et al. suggested lower postoperative FLACC scores after IT administration 0.2 mcg/ kg in children 3-12 years 47. Profound hypothermia in infants has been reported when combining IV sedation with dexmedetomidine following SA with bupivacaine and IT clonidine 48.
INDICATIONS FOR SPINAL ANESTHESIA
Typically, procedures performed under SA have a short duration and are infraumbilical. Open inguinal/umbilical hernia repair, orchidopexy and other penoscrotal procedures are common examples. The VISR published in 2006 a report on 1554 patients who underwent SA for various procedures 14. Given their significant experience in SA, they reported an expanded list of indications including neonatal congenital emergencies such as pyloromyotomy, omphalocele, meningomyelocele and gastroschisis repair 14. In the last decade, reports on the use of SA for infant orthopedic surgery including achilloplasty and polydactyly repair have emerged 42,51.
As institutions gain familiarity with the technique, the pediatric SA indications continue to expand. In the study from 2022, newer indications included umbilical hernia repair, thigh muscle biopsy and laparoscopic inguinal hernia 42. SA was effective for laparoscopic hernia repair with abdominal insufflation through the inguinal area to 10 mmHg 52. Reports show that insufflation was well tolerated, and pneumoperitoneum had no effect on ventilation 52. Notably, infants did not develop hypotension, a common response to insufflation in this age group 52. Factors leading to successful use of SA in laparoscopic surgery included pneumoperitoneum pressure < 8 mmHg, operative time < 60 minutes, and sensory level higher than T10 42,53. A 2019 case series reported laparoscopic pyloromyotomy under SA 54.
Most reports on pediatric SA address the youngest age group of neonates and infants. The use of SA in children > 3 years is limited mainly due to psycho-behavioral factors such as fear of needle, pain and difficulty to remain still during the procedure. However, some pediatric centers perform SA in older children if GA is considered high-risk 42. Sedation given pre-operatively and/or intraoperatively can be beneficial.
Reports from this past decade show that institutional protocols may help optimize pediatric SA 15. After implementation of a standardized SA protocol for infants undergoing inguinal herniorrhaphy, Chen et al. showed that post-operative time from bandaging the surgical wound to exiting the OR for SA was more efficient compared to the GA group 16. Whitaker et al. report the average time to placing SA in an infant as 3.8 ± 2.7 minutes in their institution 55. In the GAS study anesthesia time was also shorter in the SA group (51 vs. 66 minutes) 39. Islam et al. showed that in 12 patients undergoing laparoscopic pyloromyotomy, the time to recovery was significantly shorter after SA (5 vs. 26.5 min in GA group), decreased OR time, and significant cost savings 54.
Another advantage of SA is a reduction in hospital length of stay for healthy infants undergoing day surgery 15. Some hospitals report significantly shorter hospital stay of infants with SA for penoscrotal procedures as compared to GA (5.3 hours versus 17.1 hours) 15. Kokki et al. reported a median time to discharge after SA for herniotomy in patients ASA I-II aged 6 months - 10 years of 230 minutes, although 85% of children received pain medication at home for 2-3 days 56. Higher risk infants are not candidates for same day discharge. These patients include ex-premature infants less than 60 weeks and infants with lower weight or a history of recent apnea, including early postoperative apnea 56. Extended postoperative cardiorespiratory monitoring with overnight stay should be considered for ex-premature infants who are < 60 weeks old or term infants < 45 weeks old if sedation was used with SA as the risk of postoperative apnea persists 56.
COMBINED SPINAL/CAUDAL ANESTHESIA
Combined spinal/caudal anesthesia (CSCA) is an option to extend block duration for longer surgical procedures to obviate the need for airway intervention, minimize the use of opioids and avoid potential risks related to GA. Highest risk groups who may benefit from CSCA are patients with recent upper airway infection, children with congenital airway abnormalities and ex-premature infants with a history of apnea or parenchymal lung disease 57. Practitioners should be mindful of the risks of deepening sedation, over a prolonged period with an unprotected airway.
Jayanthi et al. developed a spinal/ caudal protocol in infants undergoing prolonged urologic procedures with an average time of surgery 109 minutes 57. An initial IT injection of 0.5% isobaric bupivacaine was followed by the placement of a caudal catheter. One hour after the injection, 3% chloroprocaine was administered via the caudal catheter to prolong the duration of the surgical block. Perioperative sedation with pre-operative oral midazolam and intraoperative IV dexmedetomidine was used.
Due to the higher toxicity risk in infants, caution should be taken when redosing LA. Although, a pharmacokinetic model created by Frawley et al. for predicting levobupivacaine concentration in neonates and infants supports the safety profile of a repeat spinal (levobupivacaine 1.0 mg/kg) dose, the group did recommend caution, given unpredictable AAG levels in this age group 20. In settings where SA (levobupivacaine 0.5%, 1 mg/kg) is augmented with immediate caudal blockade, a dose of 1.5 mg/kg can be used safely. If the SA is augmented one hour after a repeated dose of 2.5 mg/kg can be used in the caudal component 20.
SEDATION DURING SPINAL ANESTHESIA
In the last decade, several reports on the use of sedation to facilitate performance and extend pediatric SA duration have emerged. Sedation with intranasal (IN) dexmedetomidine (4-5 mcg/kg) one hour prior to OR admission and additional 1-2 mcg/kg of fentanyl immediately prior to OR admission in infants > 4 months allowed for a median duration of 95 minutes of urologic surgery with a maximum of 183 minutes 58. The need for additional iv sedation varied from 12% to 37% procedures 15,58. In 2019, Chiao et al. reported the use of IV dexmedetomidine infusion (1-2 mcg/kg/h) with SA, without airway manipulation, for successful laparoscopic infant inguinal hernia repair in a series of 3 patients 52. In children > 2 years, SA combined with IV dexmedetomidine infusion ± ketamine boluses can be used for lower limb surgeries where the patients are likely to have a challenging intubation (i.e. arthrogryposis, cerebral palsy or osteogenesis imperfecta) 59,60. Emerging literature suggests increased popularity of alpha-2 agonists, although the reader should remember that cardiovascular effects can be significant with bolus administration and should be weighed against the benefits of sedation, especially in neonates.
FAILED BLOCK AND OTHER COMPLICATIONS
SA success rate, defined as return of CSF through the spinal needle in the GAS study was 86.9% 24. In the VISR, SA was successful in 83% of patients when performed by resident trainees including those having their first experience with an infant LP. In the same study, SA success reached 98.9% when the attending anesthesiologist performed the LP 14. There are no established and consistent risk factors for failed SA supported by strong evidence. The incidence of bloody tap on the first attempt is considered a moderate evidence predictor 24. Performance of SA by a pediatric anesthesiologist versus a general anesthesiologist showed weak evidence favoring the former 24. The success rates of non-anesthesia personnel (i.e. neonatologists) may differ. There is no evidence that low gestational age or weight might be impactful for block failure 24.
Complications related to pediatric SA are infrequent 61,62. Post Dural Puncture Headache (PDPH) is one of the most commonly described with a reported incidence in children of up to 15% 63. It usually occurs within 3 days of a neuraxial procedure but may be delayed for as much as 2 weeks. Positional headache that resolves with supination is the classical symptom. PDPH has been reported in infants, but the incidence may be under-reported due to difficulty with clinical assessment. In children < 10 years, lower incidence of PDPH is explained by relatively low CSF pressure and increased relative stiffness of the epidural space 64,65. In the group where the criterion of documented opening pressure was applied, the investigators found the following predisposing PDPH factors: age > 10 years, female, lower body mass index, landmark based dural puncture (compared with fluoroscopy guidance), use of sedation, higher opening pressure and presence of pseudotumor cerebri 65. It is worth mentioning that PDPH may not be the only symptom 63. Children may experience nausea, vomiting, and vertigo 63,66. Transient or permanent hearing impairment after SA is described in adults, but not in children 67.
Established treatments of bedrest, IV fluids, oral or IV analgesics with caffeine have been adopted from adult studies but no pediatric literature supports these measures 68. Further, prophylactic bed rest following LP is not thought to reduce PDPH incidence. If conservative treatment fails to relieve PDPH and the patient continues to have debilitating symptoms, an epidural blood patch (EBP) can be considered 69. In adults, the blood injection needed for the patch ranges from 15-20 ml or until backpressure is felt. In 2002, Kokki et al. reported the efficacy of EBP was not correlated with higher injected blood volumes and 0.2-0.3 ml/kg volumes successfully abated PDPH in children 69. An added challenge in children is the likely need for GA for performing an EBP. This removes patient feedback as a measure when performing the EBP. There are anecdotal reports on the need for EBP after symptomatic treatment with simultaneous insertion of an epidural catheter in the event that a second EBP was required 64. The ideal timing for EBP in children has not been determined. It is advisable to delay EBP, given the risks associated with the procedure, the need for GA and the high probability of spontaneous recovery without an EBP 69.
In the last decade, a Cochrane review from 2017 in adults showed moderate quality evidence that atraumatic needles reduce PDPH risk without increasing adverse events such as paresthesia or backache 70. Similarly in children, atraumatic needles, preferably smaller size (25G) are recommended as larger cutting tip needles are associated with a higher incidence of PDPH 65,68.
WHY IS SPINAL ANESTHESIA UNDERUTILIZED IN CHILDREN?
Despite the benefits of pediatric SA, especially in ex-premature infants with cardiorespiratory disease, the technique remains underutilized accounting for less than 5% of all neuraxial techniques 62. Lack of familiarity with the technique, both during training and practice, has made many pediatric anesthesiologists reluctant to perform the procedure 43,71. Only certain institutions have adopted pediatric SA as a routine.
Increased adoption of pediatric SA requires greater exposure of the technique by both trainees and attending anesthesiologists 43. Using simulation with neonatal models could help increase the appreciation of equipment set up, positioning and assessment of risk, including distance from skin to subarachnoid space 72. Having an experienced colleague, provide hands on intraoperative teaching, including troubleshooting tips, may improve the likelihood that less experienced colleagues become more inclined to using pediatric SA, albeit with guidance in their initial experiences 43. Finally, developing a standardized pathway with the perioperative team focusing on effective and efficient use of pediatric SA could foster an environment where the use of the technique may be standardized.
SUGGESTIONS FOR FUTURE RESEARCH AND DEVELOPMENT OF SPINAL ANESTHESIA IN CHILDREN
There have been promising reports of using pediatric SA for an expanded list of indications in the last decade, but still questions remain. Finding the optimal way to enhance duration of pediatric SA through adjuvants and/or sedation without increasing the risk of adverse events requires higher quality evidence-based guidance. Evaluating such strategies in different age groups is important given the well acknowledged physiological and pharmacological differences. Furthermore, pharmacokinetic studies on LA used for pediatric SA, aside from levobupivacaine, may further help guide timeliness of redosing. Finally, further research on the role of US for pediatric SA and whether there is a pragmatic way to incorporate the technology to enhance efficacy and safety is needed.
CONCLUSIONS
Despite the increasing number of published reports on the use of pediatric SA over the last decade, it remains an underutilized mode of anesthesia accounting for only 5% of all the neuraxial techniques. Indications for SA are expanding from traditional infraumbilical surgery to more recent reports on supraumbilical and laparoscopic surgery with low insufflation pressures. These reports used supplemental IN and IV sedation to prolong SA to approximately 90 minutes. The risk of post-operative apnea persists in ex-premature infants < 60weeks and term infants < 45 weeks undergoing SA with sedation. Combined spinal/caudal anesthesia in infants can also be used to extend block duration over 90 minutes. Bupivacaine remains the most used LA for SA but promising pharmacokinetics of levobupivacaine in infants point to its safety. The use of US prior to pediatric SA can provide useful images to assess the spinal cord anatomy but evidence for use of US both pre-procedural and for real-time needle guidance is limited.











 text in
text in 



