Natural resistance to HIV-1 infection exhibited by some individuals who, despite being in direct contact with the virus, show no evidence of infection (known as HIV-1-exposed seronegative, HESN) has been associated, among others, to polymorphisms in genes that encode the HIV-1 co-receptors CCR5 and CXCR4, as well as the alternative co-receptor CCR21.
Seven major haplotypes have been described based on unique combinations of polymorphic sites in CCR5 and the adjacent CCR2 gene, which were phylogenetically established and named as human haplogroups (HH) A, B, C, D, E, F (F1, F2) and G (G1, G2)2,3. Some of these have been associated with either resistance (HHG2, HHA, HHF2) 2,4,5 or increased susceptibility to HIV-1 infection and accelerated AIDS progression (HHE) 6-8. Further-more, polymorphisms located in promoter or intronic regions of the natural ligands of CCR5, CCL3 (also known as MIP-1α, encoded by CCL3) and CCL5 (also known as RANTES, encoded by CCL5) could modify the secretion levels of these peptides further influencing the resistance or susceptibility (R/S) to HIV-1 infection by competition with the CCR5 co-receptor 3.
In this study, we determined the association of multilocus haplotypes formed by variants on the promoter and coding regions of the CCR5-CCR2 locus, and the CCL3 and CCL5 genes with R/S to HIV-1 infection in a cohort of serodiscordant couples from Colombia.
Materials and methods
Study group
Samples were taken from a cohort of serodiscordant couples previously recruited at the Hospital San Vicente Fundación in Medellín that included 49 (17 men, 32 women) HESNs and 35 (31 men, four women) seropositive (SP) individuals.The selection criteria for HESNs were previously described 9 as absence of anti-p24 antibodies during at least six months of follow-up, in which at least two sexual intercourses occurred per week with an HIV-1 positive individual. For all cases, the HIV-1 status was tested by ELISA (Abbot Axsym System) and confirmed through western blot (NewLab blot) following the manufacturer’s instructions.
This study was approved by the bioethics board of the Universidad de Antioquia’s Instituto de Investigaciones Médicas and all participants signed an informed consent.
Genotype analysis
DNA was obtained from peripheral blood using the phenol-chlorophorm method. The genotyping of the 32-base-pair deletion (Δ32, rs333) on CCR5 was performed by conventional PCR as previously described 9. The identification of SNPs in the selected genes (-2733A>G [rs2856758], -2554G>T [rs2734648], -2459G>A [rs1799987], -2135T>C [rs1799988], -2132C>T [rs41469351], -2086A>G [rs1800023] and -1835C>T [rs1800024] in CCR5; 190G>A [64Val>Ile, rs1799864] in CCR2; 73+40C >T [rs35511254] and 74-333C>T [rs111235874] in CCL3; -403G>A [rs2107538] and -28C>G [rs2280788] in CCL5) was performed by PCR-RFLP using the oligonucleotides, restriction enzymes and protocols previously reported by González, et al.2,3. PCR reactions were performed on 25 µl final mix containing 2.5 µl 10X reaction buffer (Thermo Scientific, St. Leon-Rot, Germany), 0.2 µl recombinant Taq polymerase 5 IU/µl (Thermo Scientific), 1 µl primers 25 mM and 2 µl DNA 8 µg/ml. Endonuclease digestion was performed on 13 µl of amplification products with 0.2 µl restriction endonucleases 10 IU/µl (Thermo Scientific) and 1.5 µl 10X reaction buffer (Thermo Scientific) following the protocols reported by González, et al.2,3. PCR and endonuclease digestion products were checked on 1.5% and 2.5% agarose gels, respectively.
Statistical analysis
Hardy-Weinberg equilibrium and genotype and allele frequencies were calculated using the GENPOP software, v. 3.4 10. Most probable haplotypes were generated through a maximum likelihood test using the Arlequin software, v. 3.511 and haplogroups were defined according to previous reports 2,5. The DnaSP software v.5 12 was used to calculate linkage disequilibrium and haplotype frequencies. All comparisons were performed in the Graphpad Prism 6.05 software using either the chi-square or the Fisher exact test, both with Bonferroni correction for multiple comparisons (referred to as p’), based on the number of principal haplotypes (with a frequency >0.05) as previously described 13. A corrected p’ value (p x number of comparisons per group) <0.05 was considered statistically significant.
Results
Demographic data
In this study we included samples from 49 HESN and 35 SP individuals of a previously defined cohort of serodiscordant couples (including heterosexual and homosexual couples, as well as SP individuals with more than one HESN sexual partner). In our samples, 17 males and 32 females corresponded to the HESN group, whereas 31 males and four females corresponded to the SP group. The mean age was 35 and 31 years for HESN and SP, respectively. The time of HIV-1 exposure among HESN individuals ranged between six months and four years. No genetic structure between cases and controls was expected, as the population was recruited in Medellín, whose population has been previously demonstrated to be homogeneous given its history of isolation 14,15.
Haplotype distribution
The selected loci on CCR5 and CCR2 successfully genotyped in all individuals of our cohort. These variants were successfully grouped within the nine previously reported HHs. Of all HHs, only HHB was not found in this study. In total, we found 21 out of 36 expected haplogroup pairs (binomial coefficient C(8,2)), with the presence of 14 and 17 pairs in the SP and HESN groups, respectively, C/E being the most frequent in both groups (0.257 for SPs and 0.265 for HESNs) (table 1).
Table 1 Frequencies obtained for single locus analyses. Left columns indicate the frequency of the single copy of a given haplotype, whereas the right columns indicate the frequency of paired haplotypes. Haplotypes marked in bold reached statistical significance before Bonferroni correction.
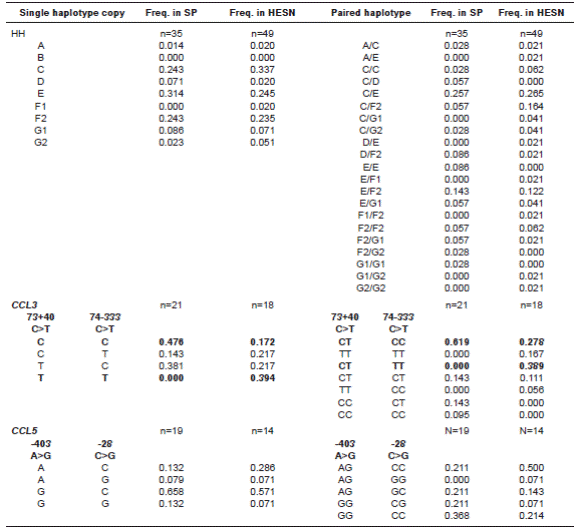
HH: human haplogroup; CCL3: C-C motif ligand 3; CCL5: C-C motif ligand 5; SP: HIV-1 seropositive; HESN: HIV-1 exposed seronegative
For the two polymorphic sites of CCL3, only 18 and 21 samples successfully amplified both SNPs in HESNs and SPs, respectively. For these, the four possible haplotypes (2 loci x 2 variants in each) and seven out of ten expected haplotype pairs (C(4,2)) were observed in total, with four and five pairs in the SP and HESN groups, respectively. For the two polymorphic sites in CCL5, only 14 and 19 samples successfully amplified both SNPs in HESNs and SPs, respectively. For these, the four possible haplotypes and five out of ten expected haplotype pairs were observed in total, with four and five pairs found in the SP and HESN groups, respectively (table1).
To determine the combined association of variants in HHs, CCL5 and CCL3 with R/S, a multilocus analysis was performed. Nonetheless, we could only use 14 samples in HESNs and 19 in SPs that successfully amplified and enzyme-digested the 13 tested loci. For this multilocus combination of HH, CCL3 and CCL5, we found 26 haplotypes and 22 haplotype pairs in total, with ten haplotypes and 12 pairs in HESNs and 16 haplotypes and 15 pairs in SPs, out of 128 expected haplotypes (8HH x 4CCL3 x 4CCL3) and 805 expected pairs (23HH x 7CCL3 x 5CCL5) (table 2).
Table 2 Frequencies obtained for multilocus haplotype analyses. Left columns indicate the frequency of the single copy of a given multilocus haplotype, whereas right columns indicate the frequency of paired multilocus haplotypes. Haplotypes marked in bold reached statistical significance before Bonferroni correction.

HH: human haplogroup; CCL3: C-C motif ligand 3; CCL5: C-C motif ligand 5; SP: HIV-1 seropositive; HESN: HIV-1 exposed seronegative
Association analysis
Whereas none of the HHs were individually associated with R/S to HIV-1 infection, homozygosis of HHE (E/E) was only found in infected individuals, showing a non-significant tendency towards susceptibility (p=0.069, p’>1). For CCL3, the haplotype C-C was higher in SPs (p=0.004, p’=0.016) while haplotype T-T was higher in HESNs (p<0.0001, p’<0.0001). When paired, the combination CT-CC was associated with susceptibility (p=0.035, p’=0.245), whereas CT-TT was associated with resistance (p=0.002, p’=0.014). For CCL5, no significant differences were found for either haplotypes or pairs of haplotypes. After multilocus analysis we observed that the simultaneous presence of HHC with T-T in CCL3 and G-C in CCL5 was strongly associated with resistance to infection (p=0.0005, p’=0.006), and so was the paired combination of HHC/F2 with TT-TT in CCL3 and AG-CC in CCL5; however, this latter situation did not remain significant after Bonferroni correction (p=0.040, p’=0.880). These results are summarized in table 3.
Table 3 Summary of single and multilocus haplotypes and pairs of haplotypes that reached statistical significance, both before and after Bonferroni correction. Frequencies in bold show the associated group, thus indicating susceptibility or resistance towards infection.
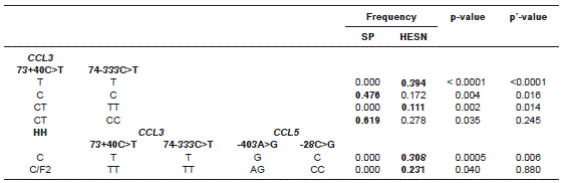
SP: HIV-1 seropositive; HESN: HIV-1 exposed seronegative; HH: human haplogroup; CCL3: C-C motif ligand 3; CCL5: C-C motif ligand 5
Discussion
An important component involved in the R/S to HIV-1 infection is the expression levels of the CCR5 co-receptor on the cell. Previous studies have shown that several members of the NF-κB and c-Rel families have different binding and affinity across the CCR5 promoter region depending of both single SNPs and whole CCR5 HH, which regulate in different ways the mRNA levels, translation capacity and, ultimately, protein expression 16. Indeed, previous findings have shown that HHE has a higher promoter activity than the ancestral and protective HHA, as measured by the luciferase activity test 17,18, leading to higher expression of CCR5 in individuals carrying HHE.
Although not significant after Bonferroni correction, probably due to our small sample size, our finding concerning HH E/E pointing towards susceptibility to HIV-1 infection is in concordance with previous studies showing an association with seroconversion and progression to AIDS 2,7,19, and could be explained by the expected higher expression of CCR5. An interesting point is that the susceptibility-associated effect of the HHE has also been reported when paired with HHG2, leading to increased viral production in vitro, even though the latter carries the Δ32 mutation, possibly due to restitution of the normal CCR5 levels over the cell membrane 20. Nevertheless, HHG2 has been effectively associated with protection and decreased seroconversion when found in the homozygous state, or when paired with either HHA or HHF2 19. These findings suggest that for HHs, the association with R/S depends on the effect of both copies carried by an individual over the overall expression of CCR5 on the cell membrane.
Our results regarding CCL3, where the T-T haplotype was associated with resistance and C-C with increased risk, contrast with previous reports where the presence of both C-C and T-T haplotypes were associated with resistance in Africans, whereas the absence of haplotype C-C in European Americans was associated to AIDS progression 3. Different ancestral backgrounds might be affecting how these variants synergize during exposure and infection. This becomes important since the Colombian population originated through admixture processes of Amerindian, European and African populations 14; therefore, a new genetic background might explain these results.
Even though we did not find an association in CCL5 alone, as was previously reported 3, we did find an interesting association when variants in CCR5/CCR2, CCL3 and CCL5 were analyzed together, with the multilocus haplotype formed by HHC, T-T in CCL3 and G-C in CCL5 associated with resistance. Although two meta-analyses 21,22, with only one single variant in CCL5 analyzed, have reported no association of any of them with R/S, our findings suggest that the simultaneous presence of variants on different genes involved in a common signaling or metabolic pathway (receptor and its ligands, in our case) can lead to a certain resistant or susceptible phenotype, probably due to the synergistic effect of these variants. We thus hypothesize that the resistance to viral infection in these cases might be due to the low expression of the viral specific co-receptors and high levels of their natural ligands competing for its use, and viceversa, in the susceptible phenotype. However, as reviewed recently 23, functional studies are required to validate the function of intronic and promoter variants in CCL3 and CCL5 mRNA levels and stability and of the secretion of MIP-1α and RANTES, as no data concerning these effects is available.
Despite the small sample size, mainly because of the exploratory characteristics of this study, the statistically significant results obtained here, even after the correction for multiple comparisons, support the accuracy of our results and suggest strong effects. Careful observations should, however, be considered, especially since the sample size of the CCL3, CCL5 and multilocus analyses fell short due to poor amplification products. Finally, to increase the strength of this study, our results should be validated in larger cohorts.














