Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.20 no.2 Bogotá May/Aug. 2015
https://doi.org/10.15446/abc.v20n2.43291
Doi: 10.15446/abc.v20n2.43291
Artículo de investigación
ALLEVIATION OF SALINITY STRESS ON Vicia faba L. PLANTS VIA SEED PRIMING WITH MELATONIN
Disminución del estrés salino en plantas de Vicia faba L. a través de la activación de las semillas con melatonina
Mona Gergis DAWOOD1, Mohamed E. EL-AWADI1.
1Botany Department, National Research Centre, 33 El-Bohooth st.(former El-Tahrir st.). Dokki, P.O. Code 12622, Cairo, Egypt.
For correspondence. monagergis@yahoo.com
Received: 13 May 2014; Returned for revision: 30 October 2014; Accepted: 13 December 2014.
Associate Editor: Hernán Mauricio Romero.
Citation / Citar este artículo como: Dawood MG, El-Awadi ME. Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta biol. Colomb. 2015;20(2):223-235. doi: http://dx.doi.org/10.15446/abc.v20n2.43291
SUMMARY
Melatonin is an environmentally friendly-molecule with a potent free radical scavenger and antioxidant capacity. Two pot experiments were conducted during two successive winter seasons (2011/2012 and 2012/2013) at the wire-house of the National Research Centre, Dokki, Cairo, Egypt to study the potentiality of melatonin (100 mM and 500 mM) in alleviating the harmful effect of diluted seawater at a relatively low and high concentrations (3.85 dS/m and 7.69 dS/m, respectively) on the performance of faba bean plants. The results revealed that irrigation of faba bean plants with diluted seawater reduced growth parameters (plant height, leaves number/plant, fresh and dry weights of plant), relative water content (RWC), photosynthetic pigments (chlorophylls a, b and carotenoids), indole acetic acid, total carbohydrate, K+,Ca+2, as well as the ratios of K+/Na+ and Ca+2/Na+. This was accompanied by significant increases in phenolic content, compatible solutes (total soluble carbohydrate, free amino acids, proline), Na+ and Cl- relative to the control plants (untreated plants). On the other hand, melatonin treatments improved growth parameters, RWC, photosynthetic pigments, total carbohydrate, total phenolic content, indole acetic acid, K+,Ca+2 as well as K+/Na+ and Ca+2/Na+ ratios, either in the plants irrigated with tap water or with diluted seawater, as compared with corresponding controls. Meanwhile, melatonin treatments reduced the levels of compatible solutes, as well as Na+ and Cl- contents, relative to those of corresponding controls. Salinity stress and/ or melatonin treatments induced the production of new protein bands that did not occur in the control plants. Melatonin at 500 mM had a more pronounced effect in alleviating the adverse effects of the two salinity levels under study on the performance of faba bean plants than 100 mM melatonin.
Keywords: indole acetic acid, legumes, minerals, N-acetyl-5-methoxytryptamine, protein patterns, seawater.
RESUMEN
La melatonina es una molécula ambientalmente amigable con una potente capacidad antioxidante y de trampa de radicales libres. Dos experimentos en materas fueron realizados en dos inviernos consecutivos (2011/2012 y 2012/2013) en instalaciones del Centro Nacional de Investigaciones, Dokki, Cairo, Egipto, para estudiar el potencial de la melatonina (100 mM and 500 mM) para disminuir los efectos nocivos del agua de mar diluida a concentraciones relativamente bajas y altas (3,85 dS/m and 7,69 dS/m, respectivamente). Los resultados mostraron que la irrigación de plantas de haba con agua de mar diluida reduce los parámetros de crecimiento (altura de la planta, número de hojas/planta, peso fresco y seco de la planta), el contenido relativo de agua (RWC), los pigmentos fotosintéticos (clorofilas a, b y carotenoides), el ácido indo lacético, los carbohidratos totales, K+, Ca+2, al igual que las relaciones K+/Na+ y Ca2+/Na+. Esto fue acompañado por un incremento significativo en el contenido de fenoles, solutos compatibles (carbohidratos solubles totales, aminoácidos libres, prolina), Na+ y Cl- en comparación con las plantas control (plantas no tratadas). De otro lado, los tratamientos con melatonina mejoraron los parámetros de crecimiento, RWC, los pigmentos fotosintéticos, carbohidratos totales, contenido fenólico total, ácido indo acético, K+,Ca+2 al igual que las relaciones K+/Na+ y Ca+2/Na+ , tanto en las plantas irrigadas con agua dulce de la llave como en las irrigadas con agua de mar diluida en comparación con los controles correspondientes. De otro lado, los tratamientos con melatonina redujeron los niveles de solutos compatibles, al igual que los contenidos de Na+ y Cl-, en comparación con los controles. El estrés por salinidad y/o los tratamientos con melatonina indujeron la producción de nuevas bandas de proteínas que no estuvieron presentes en las plantas control. El tratamiento de melatonina 500 mM tuvo un efecto más pronunciado que el tratamiento de 100 mM en disminuir los efectos adversos de los dos niveles de salinidad estudiados sobre el comportamiento de las plantas de haba.
Palabras clave: ácido indol acético, agua de mar, leguminosas, minerales, N-acetyl-5-methoxytryptamina, patrones de proteínas.
INTRODUCTION
Faba bean (Vicia faba L.) is one of the most important crops cultivated in the developing countries due to the richness of seed protein content. The importance of faba bean in Egypt lies not only as human food, animal fodder and green manure but also due to its importance in crop rotation via fixing atmospheric nitrogen that enriches the soil with nitrogen and organic matter as well as improving the water use efficiency of the cropping system (Khalafallah et al., 2008).
Fresh water resources are becoming limited due to the competition with human and industrial use. Furthermore, the saline water can vary greatly in quality depending on type and quantity of dissolved salts. In this respect, irrigation with diluted seawater plays an important role in saving fresh water resources and can be used successfully to grow crops under certain conditions (Zeid, 2011). Tolerance of plants to salinity stress is very complex at whole plant and cellular levels and involves changes in their morphology, physiology and metabolism (Ashraf and Harris, 2004). Reduction of photosynthetic activity, accumulation of organic acids and osmolytes, and changes in carbohydrate metabolism are typical physiological and biochemical responses to stress. One of the most important responses of plants to abiotic stresses is over production of different types of compatible solutes (Ashraf and Harris, 2004). Compatible solutes are low molecular weight, highly soluble compounds that are usually non-toxic at high cellular concentrations. Generally, such solutes protect plants from stress through different courses, including contribution to cellular osmotic adjustment, detoxification of reactive oxygen species, protection of membrane integrity and stabilization of enzymes and proteins (Bohnert and Jensen, 1996). Furthermore, some of these solutes are commonly referred to as osmoprotectants because they protect cellular components from dehydration injury. Bartels and Sunkar (2005) mentioned that organic solutes such as soluble carbohydrates, soluble proteins, total free amino acids and proline have been involved in osmotic regulation in plant and playing an important role in the tolerance of plants to salinity stress. Different abiotic stresses may cause osmotic stress, oxidative stress and protein denaturation in plants, which lead to similar cellular adaptive responses such as induction of stress proteins and acceleration of reactive oxygen species scavenging systems (Zhu, 2002).
Several researchers attempted to enhance the salinity tolerance of different crops through using antioxidants as pre-sowing seed treatments or exogenous application on plants at different growth stages. Priming (osmo-conditioning) is one of the physiological methods that improves seed performance and provides faster and synchronized germination and performs better under adverse conditions (Ashraf and Foolad, 2005).
Melatonin (N-acetyl-5-methoxytryptamine) was discovered in plants during 1995. It is widely present in many higher plants, dicotyledons and monocotyledons. Melatonin has been detected and quantified in roots, shoots, leaves, fruits and seeds of a considerable variety of plant species. The levels of melatonin in plant organs vary considerably, from picograms to micrograms per gram plant material. Generally, seeds and leaves have the highest level of melatonin while fruits have the lowest (Van Tassel et al., 2001, Tan et al., 2007). Many microorganisms including bacteria and fungi produce melatonin (Hardeland and Poeggeler, 2003). The decomposition of microorganisms releases melatonin into the surrounding soil and the rootlets of the plants may absorb this melatonin and recycle it. Melatonin is an indolic compound (biogenic indoleamine) structurally related with other important substances, such as tryptophan, serotonin, indole-3-acetic acid (IAA), etc. Melatonin-intermediate products show antioxidant properties and have synergistic action with other antioxidants, such as ascorbic acid, glutathione, etc. (Arnao and Hernández-Ruiz, 2009). Melatonin is soluble in both water and lipid so it may act as a universal hydrophilic and hydrophobic antioxidant (Janas and Posmyk, 2013). Several authors hypothesized that melatonin may possess some auxin- like effects (Kolár and Machackova, 2005) and may act as a regulatory molecule in plants (Van Tassel et al., 2001). Its antioxidant activity protects different plant tissues and organs, particularly reproductive tissues, fruit and germ tissues of the seed from oxidative stress due to environmental stresses, such as drought, salinity, cold, heat, ultraviolet light and ozone (Van Tassel et al., 2001). Tan et al. (2007) mentioned that elevated levels of melatonin probably protect plants against water and soil pollutants through acting as a direct free radical scavenger and as an indirect antioxidant. Melatonin directly detoxifies the hydroxyl radical, hydrogen peroxide, nitric oxide, peroxynitrite anion, peroxynitrous acid, and hypochlorous acid that are accumulated under stressful environments. Additionally, melatonin caused increases in the activity of several antioxidant enzymes, especially at harsh environments. One melatonin molecule may scavenge up to 10 free radicals (Tan et al., 2007), which contrasts with the classic antioxidants that typically detoxify one radical per molecule. Its antioxidant activity may manifest itself in several ways: (i) direct free radical scavenging, (ii) elevating the antioxidant enzyme activity, (iii) protecting antioxidant enzymes from oxidative damage, (iv) increasing the efficiency of mitochondrial transport chain and (v) reducing the generation of free radicals (Tan et al., 2010). Arnao and Hernández-Ruiz (2009) showed that melatonin content in roots increased due to stress, reaching up to six times the melatonin content of control roots and such increase probably plays an important role in the defense against stress.
Several investigators have studied the physiological role of melatonin in plants. These studies suggested that melatonin was involved in many plant functions as delaying flower induction (Kolár et al., 2003); stimulation of hypocotyl, coleoptile and root growth (Chen et al., 2009), and protection against chlorophyll degradation (Arnao and Hernández-Ruiz., 2009). Paredes et al. (2009) reported that melatonin functions in plants can be recognized into three categories: growth promoters as auxins; antioxidants for free radicals and serve as a first-line defense against oxidative stress; and other functions (signal molecules for circadian maintenance, regulation of flower development, or maintenance of developmental stages in fruit tissues).
Thus, this work aimed to study the effect of seed priming with melatonin in ameliorating the harmful effects of salinity (diluted seawater irrigation conditions) on the performance of faba bean plants.
MATERIALS AND METHODS
Materials
Seeds of faba bean (Viciafaba L. cv. Giza 461) were obtained from the Legumes Crops Research Department, Ministry of Agriculture and Land Reclamation, Egypt. Melatonin was purchased from Science Lab Company, 14025 Smith Road, Houston, Texas, USA.
Methods
Growth conditions
Two pot experiments were conducted at the wire-house of the National Research Centre, Dokki, Cairo, Egypt on the middle of November during two growing seasons (2011/2012 and 2012/2013). During this period, temperature ranged from 10–27 ºC. Relative humidity ranged from 21–87 %. Healthy faba bean seeds were selected for uniformity by choosing those of equal size and identical color. The selected seeds were washed with distilled water, sterilized with 1 % (v/v) sodium hypochlorite for approximately two min, and then washed thoroughly with distilled water. The seeds were divided into three groups, the first group was soaked with distilled water, while second and third groups were soaked with two concentrations of melatonin at 100 mM and 500 mM, respectively for 12 hours then allowed drying at room temperature (25 oC) for about 1h. Ten air-dried faba bean seeds were sown along a centre row in each pot (30 cm diameter) at a depth of 30 mm, in approx. 7 kg of clay: sand (3:1 v/v) soil. Granular ammonium sulphate (20.5 (w/w) % N) was applied at a rate of 40 kg N ha-1, and single superphosphate (15 % P2O5) was added at a rate of 60 kg P2O5 ha-1 to each pot. These doses of nitrogen and phosphorous were added and mixed thoroughly into the soil of each pot immediately before sowing.
The experiment consisted of three levels of melatonin namely 0 µM (control), 100 µM and 500 µM considered as ME0, ME1 and ME2 respectively where melatonin was dissolved in distilled water. Irrigation water consisted of two levels of diluted seawater namely 3.85 dS/m and 7.69 dS/m that were referred to as S1 and S2, respectively, whereas the control plants were irrigated with tap water (S0). Treatments were arranged in a factorial manner with six replicates for each treatment. Ten days after sowing, (DAS), faba bean seedlings were thinned leaving four uniform seedlings per pot.
Starting from day 15th, plants were irrigated with the two levels of diluted seawater mentioned above. Irrigation was carried out as follows, 3 times with diluted seawater followed by irrigation with tap water once and so on till the end of experiment.
Data recorded
Plants were sampled during vegetative stage (75 days after sowing) for measurement of some growth parameters (plant height, number of leaves /plant, fresh and dry weights of plant, and relative water content (RWC), moreover, fresh leaves were used for determination of photosynthetic pigments, endogenous indole acetic acid and electrophoretic protein bands. Oven- dried leaves (for 72 h at 70 ºC) were ground to a powder and kept in a desiccators to determine total carbohydrates, total soluble carbohydrate, total phenolic contents, total free amino acid, proline, as well as some mineral contents (Na+, K+, Ca+2, Cl-). It is imperative to mention that, data of the yield and its components as well as nutritive value of the yielded seeds especially its melatonin content will be reported in another research paper.
Measurements
Relative water content (RWC) was measured in the first fully-expanded leaf (from the top) using the method of Yamasaki and Dillenburg (1999).
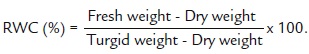
Chlorophyll a, chlorophyll b and carotenoids concentrations were estimated using the method of Moran (1982). Indole acetic acid content was determined according to Larsen et al. (1962). Fresh leaves were subjected to protein analysis according to their molecular weights by denatured sodiumdodecylsulphate (SDS)-PAGE as described by Laemmli (1970). Total soluble carbohydrates were determined according to Smith et al. (1956). Total carbohydrates were determined according to Dubois et al. (1956). Free amino acid content was determined with the ninhydrin reagent method (Yemm and Cocking, 1955). Proline was estimated according to Bates et al. (1973). Total phenolic compounds were determined according to the method described by Zhang and Wang (2001). Mineral contents of faba bean leaves (Na+, K+ and Ca+2) were determined according to Chapman and Pratt (1978) using a flame photometer. Cl- was determined according to the titration method described by Jackson (1973).
Statistical Analysis
All data were subjected to analysis of variance (ANOVA) for a randomized complete block design, after testing for homogeneity of error variances according to the procedure outlined by Gomez and Gomez (1984). Statistically significant differences between means were compared at p ≤ 0.05 using Duncan's multiple range test and presented with the standard errors.
RESULTS
Growth Parameters
All the measured growth parameters (plant height, leaves number/plant, fresh and dry weights of plant) as well as relative water content (RWC) decreased as a result of application of the two salinity levels (S1ME0 and S2ME0) relative to control plants (S0ME0) (Table 1). These decreases were significant at S2ME0 treatment, whereas, decreases due to S1ME0 treatment were significant only in plant dry weight and RWC relative to control plant. Regarding melatonin effect on plants irrigated with tap water, it was noted that both melatonin concentrations (ME1 and ME2 as 100 mM and 500 mM) increased all examined growth parameters. These increases were significant at 500 mM melatonin (ME2), meanwhile, melatonin at 100 mM (ME1) caused non-significant increases in all parameters except RWC that showed significant increase relative to control plants (S0ME0). Under salinity stress (S1 and S2), 100 mM and 500 mM melatonin (ME1 and ME2) caused increases in all the examined growth parameters relative to corresponding controls, where the increases in plant dry weight and RWC were significant. It is worthy to mention that the beneficial effects of melatonin treatments in alleviating the harmful effect of salinity stress on the growth parameters was more pronounced in the plants grown under the higher salinity level (S2= 7.69 dS/m) than those grown under lower salinity level (S1= 3.85 dS/m) relative to corresponding controls.
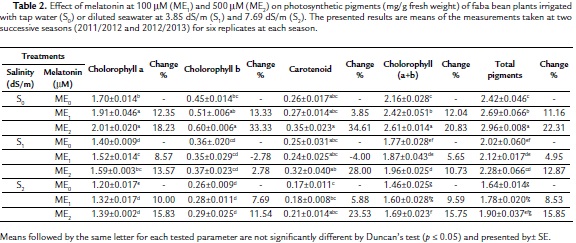
Photosynthetic Pigments
The two applied salinity levels (S1ME0 and S2ME0) caused a decrease in all components of photosynthetic pigments (chlorophylls a, b and carotenoids) and consequently chlorophyll a+b as well as total photosynthetic pigments relative to the control plants (S0ME0) (Table 2). On the other hand, chlorophyll a, a+b, and total photosynthetic pigments were significantly increased in faba bean plants irrigated with tap water under the effect of 100 mM melatonin, meanwhile 500 mM melatonin caused significant increases in all the components of photosynthetic pigments except the carotenoid content relative to the control (S0ME0). Interaction between the two salinity levels (3.85 dS/m and 7.69 dS/m) and melatonin (100 mM and 500 mM) showed that both melatonin concentrations caused significant increases in chlorophyll a and non-significant changes in chlorophyll b and carotenoids relative to corresponding controls. It is imperative to mention that the enhancement effect of 500 mM melatonin (ME2) on photosynthetic pigments was more pronounced than that by 100 mM melatonin (ME1), either in the plants irrigated with tap water (S0) or diluted seawater (S1 and S2). These increases in total photosynthetic pigments were 22.31 %, 12.87 % and 15.85 % in the plants treated with 500 mM melatonin and irrigated with tap water (S0), diluted seawater at lower (S1) and higher (S2) concentrations, respectively, as compared with corresponding controls.
Biochemical Constituents and Compatible Solutes
The two applied salinity levels (S1ME0 and S2ME0) caused significant and gradual decreases in total carbohydrate and indole acetic acid contents relative to the control plant (S0ME0) (Table 3). However, soluble carbohydrate, phenolic content, free amino acid and proline were significantly and gradually increased by increasing salinity levels relative to control plant (S0ME0). Melatonin concentrations (ME1 and ME2) in absence of salinity caused gradual increases in total carbohydrate, phenolic content and indole acetic acid accompanied by gradual decreases in soluble carbohydrate, free amino acid and proline content relative to control plant (S0ME0). Under salinity stress, the effect of melatonin on some biochemical constituents and compatible solutes of faba bean plants was more or less similar to its effect on the plants irrigated with tap water. It is worthy to mention that, 500 mM melatonin was more effective than 100 mM melatonin either in enhancement of some parameters (total carbohydrate, phenolic content and indole acetic acid) or inhibition of the others (soluble carbohydrate, free amino acid and proline).
Mineral Content
The two applied salinity levels (S1ME0 and S2ME0) caused significant and gradual increases in Na+ and Cl- percentages accompanied by significant and gradual decreases in Ca+2 and K+ percentages as well as ratios of K+/Na+ and Ca+2/Na+ relative to the control plants (S0ME0) (Table 4). Concerning melatonin effect on mineral content of faba bean plant irrigated with tap water, it was found that ME1 and ME2 caused non-significant decreases in Na+ and Cl- percentages accompanied by increases in the percentages of K+ and Ca+2 as well as ratios of K+/Na+ and Ca+2/Na+ relative to control plants (S0ME0). Furthermore, melatonin effect on mineral content of faba bean plants irrigated with two levels of diluted seawater was more or less similar to those obtained in the plants irrigated with tap water. Both melatonin concentrations caused significant decreases in Na+ and Cl- percentages accompanied by significant increases in K+ and K+/Na+ ratio, as well as non-significant increases in Ca+2 relative to corresponding controls. It was noted also that 500 mM melatonin was more effective than 100 mM in alleviating the harmful effect of the two salinity levels (3.85 dS/m and 7.69 dS/m) on the mineral content of faba bean plants.
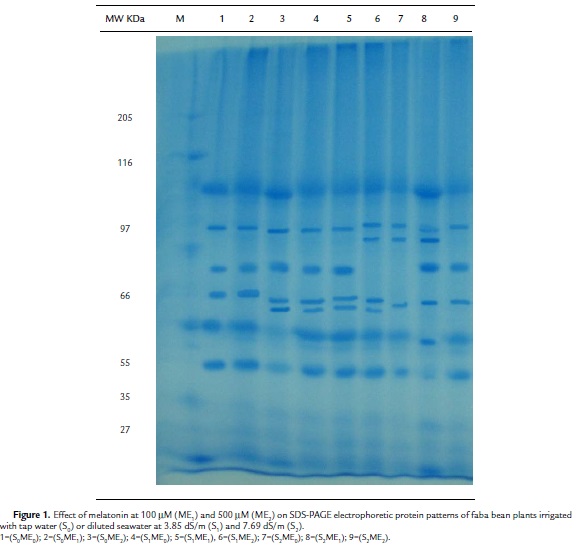
Protein Patterns
The SDS– PAGE electrophoretic protein patterns of faba bean plants grown under the effect of two different concentrations of melatonin (100 mM and 500 mM) and irrigation with either tap water (S0), diluted seawater at 3.85 dS/m (S1), or 7.69 dS/m (S2) are shown in (Table 5) and (Fig. 1).The control plants (S0ME0) exhibited 7 protein bands (Mr: 125, 103, 72, 60, 44, 35, 31 kDa, respectively). This number (7 bands) represented the least number of bands that were common among the different treatments. The high concentration of salinity (S2) alone or in combination with melatonin at the relatively low (ME1) or high (ME2) concentration induced a new protein band having a molecular rate 96 kDa. A unique protein band (Mr: 92 kDa) was induced by 500 mM melatonin (S0ME2). Furthermore, a protein band having a Mr 82 kDa seemed to be a marker for all melatonin treatments either alone (S0ME1 or S0ME2) or in combination with salinity at lower and higher levels (S1ME1, S1ME2, S2ME1, S2ME2). The low concentration of salinity (S1) alone or in combination with melatonin at the relatively low (ME1) or high (ME2) concentration induced two new protein band having a molecular rate 69 and 56 kDa.
DISCUSSION
Growth Parameters
It is well known that the responses of plant growth to salinity stress vary to different extents according to the degree and duration of stress, plant variety or species and developmental stage. In the present work, the vegetative growth parameters of faba bean plants were generally adversely affected by salinity stress (Table 1). Such a reduction in growth could be attributed to the influence of high osmotic stress and ion toxicity (Hasanuzzaman et al., 2013) or due to altered cell wall structure induced by stress (Sweet et al., 1990). Further, salinity stress might inhibit cell division, cell enlargement and expansion as mentioned by Radi et al. (2013).
The obtained data show an enhancement effect of melatonin on vegetative growth parameters of faba bean plants irrigated either with tap water or diluted seawater (Table 1). In this connection, Janas and Posmyk (2013) mentioned that exogenously applied melatonin improved developmental processes during both vegetative and reproductive growth under stress conditions. Moreover, due to its antioxidant properties, melatonin protected the roots of barley from the damaging effects of NaCl, ZnSO4 and H2O2 (Tan et al., 2010). Li et al. (2012) also reported that melatonin mediated many physiological processes in plants i.e. growth regulation and ion homeostasis and partially alleviated the salt-induced inhibition on plant growth. Melatonin has similar chemical structure as auxin-IAA so, it may play a similar role in plants as this hormone (Sarropoulou et al., 2012). Further, Hernández-Ruiz and Arnao (2008) mentioned that melatonin stimulated the vegetative growth in etiolated lupine hypocotyls in a manner similar to that of indole acetic acid. Tan et al. (2007) investigated the potential relationships between melatonin supplementation and environmental tolerance of plants. Plants having a higher content of melatonin coped better with stress under adverse environmental conditions, compared to those with lower levels of this compound (Zhang et al., 2013). Recently, there are several reports demonstrating the function of melatonin in alleviating the adverse effects of abiotic stresses (Arnao and Hernández-Ruiz, 2009, Li et al., 2012, Wang et al., 2013).
Phtosynthetic Pigments
The depressive effect of salinity stress on chlorophyll content (Table 2) may be due to the formation of proteolytic enzymes such as chlorophyllase, which is responsible for the chlorophyll degradation and /or damaging the photosynthetic apparatus (Radi et al., 2013). In addition, Santos (2004) pointed out that the decrease in chlorophyll content in severely NaCl stressed leaves was mainly due to a decrease of ALA (5-aminolinolic acid) synthesis. This acid is a precursor of protochlorophyllide, which converts to chlorophyll when exposed to light. Sabra et al.(2012) concluded that salt concentration (100 mM NaCl) reduced Chl a, Chl b and carotenoid contents in Echinacea purpurea and E. angustifolia, where that reduction was correlated with shoot Na+ content rather than Cl−, suggesting that Na+ was the major ion causing pigment reduction. Nevertheless, in other plant species like Vicia faba, the decline in the leaf chlorophyll was strictly attributed to Cl− accumulation in the leaves (Tavakkoli et al., 2010). In the present work, melatonin at two applied concentrations (100 mM and 500 mM) positively affected the photosynthetic pigments of faba bean plants either on irrigation with tap water or diluted seawater. XD et al. (2010) mentioned that treatment with melatonin played an important role in preservation of chlorophyll and promotion of photosynthesis due to raising the antioxidant enzyme activities and antioxidant contents and thus inhibiting the production of reactive oxygen species. In addition, Arnao and Hernández-Ruiz (2009) cited that melatonin treatments lowered chlorophyll degradation and slowed down the senescence process in barley plants, where 1 mM melatonin was optimal. Li et al. (2012) illustrated that pretreatment of Malus hupehensis Rehd with melatonin under high salinity conditions significantly improved plant growth and photosynthetic capacity. Zhang et al. (2013) stated that treatment with 100 μM melatonin significantly reduced chlorophyll degradation in cucumber seedlings and alleviated the effect of water stress. Furthermore, the ultrastructure of chloroplasts in water-stressed cucumber leaves was maintained after melatonin treatment. Melatonin molecule significantly reduced chlorophyll degradation and suppressed the up-regulation of senescence-associated gene and increased the photosynthetic efficiency of many plants (Tan et al., 2012, Wang et al., 2013).
Biochemical Constituents and Compatible Solutes
a. Total carbohydrates
Carbohydrates are supplied mainly through the process of photosynthesis and photosynthetic rates are usually lower in plants exposed to salinity and especially to NaCl (Ashraf and Harris, 2004). Hence, the reduction in photosynthetic pigments in faba bean leaves under the effect of salinity stress (Table 2) might have led to decreased levels of photo-assimilates in the leaves, mainly total carbohydrates (Table 3). Most of the plants are sensitive to salt stress, and high level of salinity caused reduction in carbohydrates (Hassanein et al., 2009). On the other hand, the melatonin enhancement effects on photosynthetic pigments that have been recorded in the present work might interpret the overproduction of total carbohydrates, and thus the concomitant enhancement of plant growth.
b. Indole acetic acid
The decreases in indole acetic acid (IAA) under the effect of salinity stress (Table 3) were concurrent with the decrease in vegetative growth parameters (Table 1). The reduced IAA levels under salinity stress might be attributed to the inhibition in biosynthesis of indole acetic acid and/ or increases in their degradation or transformation into inactive form. However, recent work indicated that the decreased auxin contents under salinity might be rather complicated as being underplayed by stress-responsive transcription factors and microRNAs that modulate auxin- and environment-mediated root development (Kazan, 2013). On the other hand, the promotive effect of melatonin on IAA was confirmed by Chen et al. (2009) who reported that melatonin doses increased indole acetic acid. In addition, Posmyk et al. (2009) mentioned that hydro-priming of cucumber seeds with melatonin increased IAA content.
Phenolic compounds
In response to various environmental stresses such as salinity stress, plants have developed different physiological and biochemical mechanisms to adapt or to tolerate stress. Table 3 shows that salinity stress and/ or melatonin treatments enhanced the phenolic content. Salinity induced disturbances in the metabolic process leading to the increase in the synthesis of phenolic compounds (Keutgen and Pawelzik, 2009). Actually, the accretion of reactive oxygen species (ROS) under salt stress is generally coupled with changes in net carbon gain which may strongly affect the biosynthesis of carbon-based secondary compounds, particularly leaf polyphenols (Radi et al., 2013). Phenolic compounds play an important role as antioxidants in scavenging free radicals arising from their high reactivity as hydrogen or electron donors, to stabilize and delocalize the unpaired electron (chain-breaking function), and from their ability to chelate transition metal ions (Huang et al., 2005). Beneficial effects of melatonin may also result from its signaling function, through the induction of different metabolic pathways and stimulate the production of various substances, preferably operating under stress (Tan et al., 2012). Szafranska et al. (2012) showed that melatonin added to Vigna radiata L. seeds protected the roots of chilled seedlings after re-warming and increased the synthesis of phenolic compounds, particularly derivatives of p-coumaric acid.
Compatible solutes
In the present work, salinity stress caused increase of compatible solutes, whereas melatonin treatments decreased them (Table 3). The osmotic adjustment in plants subjected to salt stress occurs by the accumulation of high concentrations of osmotically active compounds known as compatible solutes such as proline, glycinebetaine, soluble sugars, free amino acids and polyamines (Jagesh et al., 2010). These authors revealed that such substances play an important role in the adaptation of cells to various adverse environmental conditions through raising osmotic pressure in the cytoplasm, stabilizing proteins and membranes, and maintaining the relatively high water content obligatory for plant growth and cellular functions.
Proline accumulation is considered as an indicator in several plant species under salt stress conditions, acting as an osmotic protectant and contributing to the turgor maintenance of cells (Jagesh et al., 2010). Proline was involved in the synthesis of key proteins that are necessary for stress responses (Iyer and Caplan, 1998). Further, the increase in proline content could be attributed to a decrease in proline oxidase activity under saline conditions (Roodbari et al., 2013). The free amino acid accumulation associated with stress may actually be a part of an adaptive process contributing to osmotic adjustment (Dubey, 1994).
Elevated sugar levels in salt stressed plants may contribute to the turgor maintenance and stabilization of cellular membranes (Jouve et al., 2004).The accumulation of soluble carbohydrates in plants has been widely reported as a response to salinity despite a significant decrease in net CO2 assimilation rate (Murakeozy et al., 2003). According to Bohnert and Jensen (1996) carbohydrates may act as ROS scavengers and contribute to increase in membrane stabilization.
On the other hand, melatonin is a free radical scavenger and broad-spectrum antioxidant that might directly eliminate ROS when produced under stressful conditions. The accumulation of ROS under stress was inhibited by melatonin applications due to direct scavenging and/or enhanced activities of antioxidant enzymes (Tan et al., 2000, Tan et al., 2007). The results of the present work show that melatonin treatments decreased the harmful effect of salinity on faba bean plants and increased its salinity tolerance.
Mineral Content
The decreases in K+ and Ca2+ were accompanied by increases in Na+ and Cl- in faba bean leaves under the effect of salinity levels (Table 4). This conclusion agrees with those reported by Cramer (1997) and Taïbi et al. (2012) who revealed that plants exposed to NaCl take up high amounts of Na+, whereas the uptake of K+ and Ca2+ is significantly reduced. K+/Na+ ratio in plants under saline conditions is considered as one of the important selection criteria for salt tolerance (Ashraf and Harris, 2004). Tester and Davenport (2003) pointed out that low K+/Na+ ratios could disrupt protein synthesis in the cell, given that Na+ competes with K+ for binding sites essential for cellular function, but cannot substitute for K+ to activate functional enzymes. Furthermore, the maintenance of Ca2+ acquisition and transport under salinity constitutes an important determinant of salinity tolerance (Unno et al., 2002), making plants to be less susceptible to osmotic and specific ion injury. Taïbi et al. (2012) mentioned that Na+ and Ca2+ can enter cells through ion channels. These channels may control the transport of cations to the xylem and therefore, may control cation transport to the shoot. In some cases, these channels are more selective for Na+ than K+ and the increase in Ca+2 concentration reduces Na+ conductance through these channels. K+ also moves through the Ca2+ channels and can interfere with Ca2+ transport (Piñeros and Tester, 1997). Chlorine is widespread in the nature, and plants easily absorb chloride ions. Chloride ions play an important role in PSII, membrane potential stabilization and turgor and pH regulation. External Cl- concentrations higher than 20 mM can be toxic in susceptible plant species, whereas in tolerant species external concentrations four to five times higher may show no effect on plant growth (Broadley et al., 2012).
Melatonin treatments partially alleviated the harmful effect of salinity stress on mineral content of faba bean leaves (Table 4). Li et al. (2012) mentioned that melatonin might control the expression of ion-channel genes under salinity, which may possibly contribute to the maintenance of ion homeostasis and thus, improves salinity resistance in plants. The ability to limit Na+ transport into the shoots and to reduce Na+ accumulation in the rapidly growing shoot tissues is critically important for maintenance of high growth rates and protection of metabolic processes in elongating cells from the toxic effects of Na+ (Roodbari et al., 2013). Enhanced uptake of K+ and/or Ca2+ at the cost of reduced uptake Na+ in the cells of salt stressed plants is considered vital for maintaining high cellular K+/Na+ and Ca+2/Na+ ratios. Significant ameliorative effects of Ca2+ on Na+ toxicity have been reported to improve water transport (Knight et al., 1997), and growth of plants (White and Broadley, 2003).
Protein Pattern
The results of the present work showed appearance of new protein bands in faba bean plants under the effect of salinity stress and/ or melatonin treatments (Table 5 and Fig. 1). The most important mechanism involved in cell protection against salt stress is the induction of de novo synthetic protein groups (Kermode, 1997). These stress proteins may provide a storage form of nitrogen that may be re-utilized when stress is over (Badr et al., 1998). It is suggested that these new proteins may play an important role in triggering a special system that help the whole plant against salinity stress. These proteins may have an osmoprotection function (Dure, 1993) or protecting cellular structures (Close and Lammers, 1993). Shukry and El Bassiouny (2002) reported that salinization induced de novo synthesis of some salt responsive proteins of Vicia faba seeds during germination and the salt responsive proteins might be osmotin, dehydrin and ubiquitin.
Moreover, the melatonin- induced new protein bands, shown in the present work, might be attributed to the synthesis of new polypeptides or might represent degradative product(s) of proteins due to the effect of hydrolytic enzymes on high molecular weight proteins. This assumption might be reinforced by that of Posmyk et al. (2009) who reported that although melatonin protected membrane structure against peroxidation during chilling, excessive melatonin levels (∼4 μg/g fresh weight) in cucumber seeds provoked oxidative changes in proteins.
CONCLUSION
It could be concluded that melatonin treatments (100 mM and 500 mM) improved growth parameters, relative water content, photosynthetic pigments, total carbohydrate, total phenolic content, indole acetic acid, K+,Ca+2 and reduced the levels of compatible solutes, Na+ and Cl- contents in leaf tissues of faba bean plants irrigated with diluted seawater (3.85 dS/m and 7.69 dS/m). Melatonin at 500 mM had a more pronounced effect in alleviating the adverse effects of the two salinity levels on the performance of faba bean plants than 100 mM melatonin.
REFERENCES
Arnao M, Hernández-Ruiz J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J Pineal Res. 2009;46(1):58-63. Doi: http://dx.doi.org/10.1111/j.1600-079X.2008.00625.x. [ Links ]
Ashraf M, Foolad M. Pre-sowing seed treatment -a shotgun approach to improve germination, growth and crop yield under saline and non-saline conditions. Advan Agron. 2005;88:223-271. Doi: http://dx.doi.org/10.1016/S0065-2113(05)88006-X. [ Links ]
Ashraf M, Harris P. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3-16. Doi: http://dx.doi.org/10.1016/j.plantsci.2003.10.024. [ Links ]
Badr A, Haider A, Badr S, Radwan S. Genotypic variation within Egyptian wheat in response to salt stress and heat shock. Proceeding of the International Congress on Molecular Genetics. 1998;1:11-18. [ Links ]
Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 2005;166:117-123. Doi: 10.1080/07352680590910410. [ Links ]
Bates LS, Waldan RP, Teare LD. Rapid determination of free proline under water stress studies. Plant Soil. 1973;39(1):205-207. Doi: http://dx.doi.org/10.1007/BF00018060. [ Links ]
Bohnert HJ, Jensen RG. Strategies for engineering water stress tolerance in plants. Trends Biotechnol. 1996;14(3):89-97. Doi: http://dx.doi.org/10.1016/0167-7799(96)80929-2. [ Links ]
Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F. Function of Nutrients: Micronutrients. In: Marshner P, editor. Marschner's Mineral Nutrition of Higher Plants, Third Edition, Academic Press Elsevier, London, UK; 2012. p.191-248. Doi: http://dx.doi.org/10.1016/B978-0-12-384905-2.00007-8. [ Links ]
Chapman HD, Pratt PF. Methods of Analysis for Soils, Plant and Water. Univ. California. Div Agric Sci Publ. 1978;4034:162-165. [ Links ]
Chen Q, Qi WB, Reiter RJ, Wei W, Wang BM. Exogenously applied melatonin stimulates root growth and raises endogenous indole acetic acid in roots of etiolated seedlings of Brassica juncea. J Plant Physiol. 2009;166(3):324-328. Doi: http://dx.doi.org/10.1016/j.jplph.2008.06.002. [ Links ]
Close TJ, Lammers PJ. An osmotic stress protein of cyanobacteria is immunologically related to plant dehydrins. Plant Physiol. 1993;101(3):773-779. Doi: http://dx.doi.org/10.1104/pp.101.3.773. [ Links ]
Cramer GR. Uptake and Role of Ions in Salt Tolerance. In: Jaiwal PK, Singh RP, Gulati A, editors. Strategies for Improving Salt Tolerance in Higher Plants, Oxford & IBH Publishing Co. Pvt. Ltd., New Delhi; 1997. p. 55-86. [ Links ]
Dubey RS. Protein Synthesis by Plants Under Stressful Conditions. In: Pessarkli M, editor. Hand book of Plant and Crop Stress, Marcel Decker Inc, New York; 1994. p.277-299. Doi: 10.1201/9780824746728.ch16. [ Links ]
Dubois M, Guilles KA, Hamilton J.K, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350-356. Doi: http://dx.doi.org/10.1021/ac60111a017. [ Links ]
Dure L. Structural Motifs in LEA Proteins. In: Plant Responses to Cellular Dehydration during Environmental Stress. Close TJ, Bray EA, editors. Current topics in plant physiology. Plant Physiol. 1993;10:91-103. [ Links ]
Gomez KA, Gomez AA. Statistical Procedures for Agricultural Research. John Wiley and Sons Inc., Singapore; 1984. p. 680. [ Links ]
Hardeland R, Poeggeler B. Non vertebrate melatonin. J Pineal Res. 2003;34(4):233-241. Doi: http://dx.doi.org/10.1034/j.1600-079X.2003.00040.x. [ Links ]
Hasanuzzaman M, Nahar K, Fujita M. Plant Response to Salt Stress and Role of Exogenous Protectants to Mitigate Salt-induced Damages. In: Ahmad P, Azooz MM, Prasad MNV, editors. Ecophysiology and Responses of Plants Under Salt Stress. New York: Springer; 2013p. 25- 87. Doi: http://dx.doi.org/10.1007/978-1-4614-4747-4_2. [ Links ]
Hassanein RA, Bassouny FM, Barakat DM, Khalil RR. Physiological effects of nicotinamide and ascorbic acid on Zea mays plant grown under salinity stress. 1- Changes in growth, some relevant metabolic activities and oxidative defense systems. Res J Agric Biol Sci. 2009;5:72-81. [ Links ]
Hernández-Ruiz J, Arnao MB. Melatonin stimulates the expansion of etiolated lupine cotyledons. Plant Growth Reg. 2008;55(1):29-34. Doi: http://dx.doi.org/10.1007/s10725-008-9254-y. [ Links ]
Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric. Food Chem. 2005;53(6):1841-1856. Doi: http://dx.doi.org/10.1021/jf030723c. [ Links ]
Iyer S. Caplan A. Products of proline catabolism can induce osmotically regulated genes. Plant Physiol. 1998;116(1):203-211. Doi: http://dx.doi.org/10.1104/pp.116.1.203. [ Links ]
Jackson ML. Soil Chemical Analysis. 1st Edition. Prentice Hall of India Pvt. Ltd., New Delhi, India; 1973. p. 61-73. [ Links ]
Jagesh K, Tiwari AD, Munshi RK, Raghu N, Pandey Ajay AJS, Bhat AKS. Effect of salt stress on cucumber: Na+/ K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol Plant. 2010;32(1):103-114. Doi: http://dx.doi.org/10.1007/s11738-009-0385-1. [ Links ]
Janas KM, Posmyk MM. Melatonin, an under estimated natural substance with great potential for agricultural application. Acta Physiol Plant. 2013;35(12):3285-3292. Doi: http://dx.doi.org/10.1007/s11738-013-1372-0. [ Links ]
Jouve L, Hoffmann L, Hausman JF. Polyamine, carbohydrate and proline content changes during salt stress exposure of Aspen (Populus tremula L.): involvement of oxidation and osmoregulation metabolism. Plant Biol. 2004;6(1):74-80. Doi: http://dx.doi.org/10.1055/s-2003-44687. [ Links ]
Kazan K. Auxin and the integration of environmental signals into plant root development. Ann Bot. 2013;112(9):1655-1665. Doi: http://dx.doi.org/10.1093/aob/mct229. [ Links ]
Kermode AR. Approaches to elucidate the basis of desiccation-tolerance in seeds. Seed Sci Res. 1997;7(2):75-93. Doi: http://dx.doi.org/10.1017/S0960258500003421. [ Links ]
Keutgen AJ, Pawelzik E. Impacts of NaCl stress on plant growth and mineral nutrient assimilation in two cultivars of strawberry. Environ Experi Bot. 2009;65(2-3):170-176. Doi: http://dx.doi.org/10.1016/j.envexpbot.2008.08.002. [ Links ]
Khalafallah A, Tawfik KM, Abd El-Gawad ZA. Tolerance of seven faba bean varieties to drought and salt stresses. Res J Agric Biol Sci. 2008;4(2):175-186. [ Links ]
Knight H, Trewavas AJ, Knight MR. Calcium signaling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12(5):1067-1078. Doi: http://dx.doi.org/10.1046/j.1365-313X.1997.12051067.x. [ Links ]
Kolár J, Johnson CH, Machácková I. Exogenously applied melatonin (N-acetyl-5-methoxytryptamine) affects flowering of the short-day plant Chenopodium rubrum. Physiol Plant. 2003;118(4):605-612. Doi: http://dx.doi.org/10.1034/j.1399-3054.2003.00114.x. [ Links ]
Kolár J, Machácková I. Melatonin in higher plants: occurrence and possible functions. J Pineal Res. 2005;39(4):333-341. Doi: http://dx.doi.org/10.1111/j.1600-079X.2005.00276.x. [ Links ]
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. Doi: http://dx.doi.org/10.1038/227680a0. [ Links ]
Larsen PA, Harbo S, Klungron, Ashein TA. On the biosynthesis of some indole compounds in Acetobacter xylinum. Physiol Plant. 1962;15(3):552-565. Doi: http://dx.doi.org/10.1111/j.1399-3054.1962.tb08058.x. [ Links ]
Li C, Wang P, Wei Z, Liang D, Liu C, Yin L. et al. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res. 2012;53(3):298-306. Doi: http://dx.doi.org/10.1111/j.1600-079X.2012.00999.x. [ Links ]
Moran R. Formula for determination of chlorophyllous pigments extracted with N.N. dimethylformamide. Plant Physiol. 1982;69(6):1371-1381. Doi: http://dx.doi.org/10.1104/pp.69.6.1376. [ Links ]
Murakeozy EP, Nagy Z, Duhaz E C, Bouchereau A, Tuba Z. Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J Plant Physiol. 2003;160(4):395-401. Doi: http://dx.doi.org/10.1078/0176-1617-00790. [ Links ]
Paredes SD, Korkmaz A, Manchester LC, Tan DX. Reiter RJ. Phytomelatonin: a review. J Exp Bot. 2009;60(1):57-69. Doi: http://dx.doi.org/10.1093/jxb/ern284. [ Links ]
Piñeros M, Tester M. Calcium channels in higher plant cells: selectivity, regulation and pharmacology. J Exp Bot. 1997;48:551- 577. Doi: http://dx.doi.org/10.1093/jxb/48.Special_Issue.551. [ Links ]
Posmyk MM, Batabusta M, Wieczorek M, Sliwinska E, Janas KM. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling. J Pineal Res. 2009;46(2):214-223. Doi: http://dx.doi.org/10.1111/j.1600-079X.2008.00652.x. [ Links ]
Radi AA, Farghaly FA, Hamada AM. Physiological and biochemical responses of salt-tolerant and salt-sensitive wheat and bean cultivars to salinity. J Biol Earth Sci. 2013;3(1):72-88. [ Links ]
Roodbari N, Roodbari S, Ganjali A, Nejad FS, Ansarifar M. The effect of salinity stress on growth parameters and essential oil percentage of peppermint (Mentha piperita L.). Int J Adv Biol Biom Res. 2013;1(9):1009-1015. [ Links ]
Sabra A, Daayf F, Renaulta S. Differential physiological and biochemical responses of three Echinacea species to salinity stress. Scient Horticul. 2012;135:23-31. Doi: http://dx.doi.org/10.1016/j.scienta.2011.11.024. [ Links ]
Santos CV, Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Scient Horticul. 2004;103(1):93-99. Doi: http://dx.doi.org/10.1016/j.scienta.2004.04.009. [ Links ]
Sarropoulou VN, Therios IN, Dimassi-Theriou KN. Melatonin promotes adventitious root regeneration in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus x P. canescens), and MxM 60 (P. avium x P. mahaleb). J Pineal Res. 2012;52(1):38-46. Doi: http://dx.doi.org/10.1111/j.1600-079X.2011.00914.x. [ Links ]
Shukry WM, El-Bassiouny HM, Gibberellic acid effects on protein pattern, hydrolytic enzyme activities and ionic uptake during germination of Vicia faba in sea water. Acta Bot Hung. 2002; 44: 145-162. Doi: http://dx.doi.org/10.1556/ABot.44.2002.1-2.11. [ Links ]
Smith F, Gilles MA, Hamilton JK, Godees PA. Colorimetric method for determination of sugar related substances. Anal Chem. 1956;28(3):350-356. Doi: http://dx.doi.org/10.1021/ac60111a017. [ Links ]
Sweet WJ, Morrison JC, Labaritch JM, Matthews MA. Altered synthesis and composition of cell wall of grapevines Vitis vinifera L. during expression and growth inhibiting water deficits. Plant Cell Physiol. 1990;31:407-414. [ Links ]
Szafranska K. Glinska S, Janas KM. Changes in the nature of phenolic deposits after re-warming as a result of melatonin pre- sowing treatment of Vigna radiata seeds. J Plant Physiol. 2012;169(1):34-40. Doi: http://dx.doi.org/10.1016/j.jplph.2011.08.011. [ Links ]
Taïbi Kh, Taïbi F, Belkhodja M. Effects of external calcium supply on the physiological response of salt stressed bean (Phaseolus vulgaris L.). Gen Plant Physiol. 2012;2(2-4):177-186. [ Links ]
Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S,et al. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot. 2012;63:577-597. Doi: http://dx.doi.org/10.1093/jxb/err256. [ Links ]
Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM. et al. The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol Rev. 2010;85:607-623. [ Links ]
Tan DX, Manchester LC, Reiter RJ. Plummer BF, Limson J, Weintraub ST, et al. Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radic Biol Med. 2000;29(11):1177-1185. Doi: http://dx.doi.org/10.1016/S0891-5849(00)00435-4. [ Links ]
Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species?. J Pineal Res. 2007;42(1):28-42. Doi: http://dx.doi.org/10.1111/j.1600-079X.2006.00407.x. [ Links ]
Tavakkoli E, Rengasamy P. Mc Donald GK. High concentrations of Na+ and Cl- ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exper Bot. 2010;61(15):4449-4459. Doi: http://dx.doi.org/10.1093/jxb/erq251. [ Links ]
Tester M. Davenport R, Na+ tolerance and Na+ transport in higher plants. AnnBot. 2003;91(5):503-527. Doi: http://dx.doi.org/10.1093/aob/mcg058. [ Links ]
Unno H, Maeda Y, Yamamoto S, Okamoto M, Takenaga H. Relationship between salt tolerance and Ca retention among plant species. Japan J Soil Sci Plant Nut. 2002;73: 718-725. [ Links ]
Van Tassel DL, Roberts N, Lewy A, O'Neill SD. Melatonin in plant organs. J Pineal Res. 2001;31(1):8-15. Doi: http://dx.doi.org/10.1034/j.1600-079X.2001.310102.x. [ Links ]
Wang P, Sun X, Li C, Wei Z, Liang D, Ma F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res. 2013;54(3):292-302. Doi: http://dx.doi.org/10.1111/jpi.12017. [ Links ]
White PJ. Broadley MR. Calcium in Plants. Ann Bot. 2003;92(4):487-511. Doi: http://dx.doi.org/10.1093/aob/mcg164. [ Links ]
XD X, Sun Y, Sun B, Zhang J. Guo XQ. Effects of exogenous melatonin on active oxygen metabolism of cucumber seedlings under high temperature stress. Ying Yong Sheng Tai Xue Bao. 2010;21(5):1295-1300. [ Links ]
Yamasaki S, Dillenburg LC. Measurements of leaf relative water content in Araucaria angustifolia. R Bras Fisiol Veg. 1999;11(2):69 -75. [ Links ]
Yemm EW, Cocking EC. The determination of amino acids with ninhydrin. Analyst. 1955;80:209-214. Doi: http://dx.doi.org/10.1039/an9558000209. [ Links ]
Zeid IM. Alleviation of seawater stress during germination and early growth of barley. Inter. J Agric Res Rev. 2011;1(2):59-67. [ Links ]
Zhang N, Zhao B, Zhang HJ, Weeda S, Yang Ch, Yang ZC, et al. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res. 2013;54(1):15-23. Doi: 10.1111/j.1600-079X.2012.01015.x [ Links ]
Zhang W, Wang SY, Antioxidant activity and phenolic compounds in selected herbs. J Agri Food Chem. 2001;49(11):5165-5170. Doi: http://dx.doi.org/10.1021/jf010697n. [ Links ]
Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol. 2002;53:247-273. Doi: http://dx.doi.org/10.1146/annurev.arplant.53.091401.143329. [ Links ]