INTRODUCTION
Cachama blanca (Piaractus orinoquensis), is a new species reported for the South American Orinoquia previously classified as Piaractus brachypomus Cuvier, but that differs from the latter in its morphological characteristics and mitochondrial DNA (Escobar et al., 2019). Natural of the hydrographic basin of the Orinoco River that includes the Colombian Eastern Plains and much of the Venezuelan territory (Gassón, 2002), it is a distinctive species of Colombian fish farming that contributed 17%of the reported fish production in Colombia in 2018 (Ministerio de Agricultura y Desarrollo Rural [MADR] Pesqueras y Acuícolas, 2019). Some studies related to reproduction and seminal cryopreservation have been reported in this species under the P. brachypomus classification (Navarro et al., 2004; Ramirez-Merlano et al., 2011; Suárez-Martínez et al., 2019). However, according to the origin of the breeders, these reports correspond to P. orinoquensis. For this reason, considering the geographic space from which the specimens of the present study come, the taxonomic classification P. orinoquensis proposed by Escobar et al. (2019) will be adopted. Although in P. orinoquensis captive breeding package is fully standardized, reproductive seasonality and asynchronism in male and female gonadal maturation are still limiting the optimization of juvenile production (Cruz-Casallas et al., 2011). Seminal cryopreservation has played an important role in enhancing the reproduction of these asynchronous species, allowing a constant use of semen throughout the year for artificial semination processes (Ramírez-Merlano et al., 2010; Medina-Robles et al., 2019). Several works have contributed to the standardization of protocols for seminal cryopreservation in Cachama blanca evaluating packaging systems, cryoprotectants, freeze-thaw curves, and fertility rates (Navarro et al., 2004; Ramirez-Merlano et al., 2011; de Almeida et al., 2012; Moreira et al., 2012), which has allowed its use on a commercial scale. In characiforms, sperm motility activation is triggered by a hypoosmotic medium, often independently of ions or constituents of the activation media. The most frequently used activators are those constituted by SC, SB, and glucose. Osmolality ranges between 0 and 310 mOsm kg-1 have been reported in characiforms (Martínez et al., 2011; Gonçalves et al., 2013). Commercial 0.9 % SC solutions are used as an alternative to activate cryopreserved semen in field production situations. These solutions are easily accessible in the drugstore and have an osmolality of 308 mOsm kg-1 (https://ecatalog.baxter.com). Generally, it is recommended to use thawed semen immediately, since the delay in time reduces the success of fertilization significantly (Stoss & Holtz, 1981; Lahnsteiner et al., 1996). Studies carried out in Persian sturgeon Acipenser persicus Borodin showed that in the cryopreserved semen after 60 min post-thawing, sperm motility, duration of motility, fertilization, and hatching rate decreased significantly compared to fresh sperm and 30 min post-thaw semen (Aramli and Nazari, 2014). In contrast, the motility of cryopreserved sperm of brook trout Salvelinus fontinalis (Nynca et al., 2015) and Caspian trout Salmo trutta caspius (Golshahi et al., 2015) was maintained up to 60 min after defrosting without significant differences with time 0 min. Similarly, Horváth et al. (2015) found no significant differences in motility, fertilization rate, and post-thaw semen hatching at 0 and 60 min in several species of salmonids (Thymallus thymallus, Oncorhynchus mykiss, Salmo marmoratus, and Salmo trutta m. fario). Sperm changes after post-thaw storage time affect motility, cell metabolism, and the structure of plasma membrane and organelle (Babiak et al., 2001; Cabrita et al., 2010). The plasma membrane is highly sensitive to cold-shock damage, osmotic stress, and the presence of reactive oxygen species (ROS) generated during the freezing/ thawing processes (Cabrita et al., 1999). To our knowledge there are no experiments on the effect of post-thawing storage time of cryopreserved semen on sperm viability in neotropical fishes and no reports of fertility rates of semen after long-term storage. Potential post-thaw pH variations associated with sperm metabolism, membrane oxidation process, and changes in extender organics such as egg yolk and glucose have not been assessed. Consequently, the main goal of the present study was to evaluate the effect of post-thawing storage in long-term cryopreserved semen of P. orinoquensis on sperm motility, motility duration, sperm membrane integrity, pH, and fertilization rate, using two activating solutions (1 % NaHCO3 and 0.9 % NaCl).
MATERIALS AND METHODS
Fish Handling
All procedures were performed by the guidelines and recommendations for the use of laboratory animals, described by the Committee on Care and Use of Laboratory Animals, attached to the US National Research Council (Guide for the Care and Use of Laboratory Animals, 2011) and the Bioethics Committee of the University of the Llanos.
Biological material and osmolality of the samples
P. orinoquensis semen cryopreserved in 4 mL plastic macrotubes (240 x 5 mm, IMV Technologies, Minneapolis, Minnesota, USA) obtained from five sexually mature males (3.04 ± 0.3 kg), with sperm motility higher than 90 % (Ramirez-Merlano et al., 2011) was used. This semen was kept in liquid nitrogen tanks for a period of seven years in the Laboratory of Reproduction and Cryopreservation of the Institute of Aquaculture at the University of the Llanos (IALL). Before freezing, fresh sperm (with an osmolality of 287.2 ± 18.3 mOsm kg-1) was mixed with a diluent composed of10 % v/v dimethylsulfoxide, (DMSO, Sigma Chemical Co., St. Louis, Missouri, USA), 5.5 % w/v glucose (348 mOsm kg-1), (Merck, Darmstadt, Germany) and 12 % egg yolk in a 1:4 ratio (semen: diluent) (Ramirez-Merlano et al., 2011) and frozen in liquid nitrogen vapors using a CP100 dry shipper tank (Taylor-Wharton, Theodore, Alabama, USA) for 30 minutes from 28 to -100°C at an average freezing rate of 28.6°C/min (Medina-Robles et al., 2007), and subsequently immersed in liquid nitrogen in an HC 35 cryogenic tank (Taylor-Wharton, Theodore, Alabama, USA). Osmolality (mOsm kg-1) in seminal plasma was determined according to the methodology described by Ramirez Merlano et al. (2011) using an OSMETTE III Model 5010 automatic osmometer (Precision Systems Inc., Natick, Massachusetts, USA). 10 µL aliquots were used for the 5.5 % glucose solution and the activator solutions, each measurement was made in triplicate using distilled water as the control solution. Egg yolk osmolality cannot be determined by this instrumental method due to the high molecular weight of the proteins present in this matrix.
Experimental design
To evaluate the effect of post-thaw storage time (PST) and activator solution on the quality of P. orinoquensis cryopreserved semen, a completely randomized design was used in a 2 x 4 factorial arrangement consisting in two activating substances (1 % NaHCO3 [SB, 185 mOsm kg-1] and 0.9 % NaCl [SC, 308 mOsm kg-1]) and four PST (0, 15, 30 and 60 min). Sperm motility (%), duration of motility (s), and fertility (%) were taken as response variables. For variables sperm membrane integrity - SMI (%) and pH, a randomized arrangement was implemented, under the same four times described above. Three macrotubes were thawed per male (total = 15).
Evaluation of spermatic parameters
The semen was thawed in a water bath (Lab Companion DW-10H, Korea) at a temperature of 35°C, for 90 s (Ramirez-Merlano et al., 2011) and kept in sterile glass tubes at laboratory temperature (24.5 ± 0.3°C) through different PST's. The temperature was determined by a digital hygro-thermometer clock (Extech Instruments Inc., Waltham, Massachusetts, USA) and recorded every 15 min until the end of the evaluation.
Sperm motility and duration of sperm motility (DM)
Sperm motility was evaluated subjectively by the same person to reduce the variability of the analysis, using a scale of 0 % - 100 % and activating an aliquot of semen (20 µL) with 180 µL of each of the two activating substances (1:10) and using an excavated glass slide (1.0-1.2 mm deep, Micro Slides Premiere, China) placed in an optical microscope PRIMO STAR (Carl Zeiss LLC, Göttingen, Germany) at 400X magnification (Ramirez-Merlano et al., 2011; Suárez-Martínez et al., 2019). Immediately after sperm activation, the DM was evaluated, until the immobility of 90 % of the spermatozoa using a chronometer and optical microscope (400X).
Sperm membrane integrity (SMI)
This variable was determined by a supravital staining technique because spermatozoa with plasma membranes that did not maintain their integrity are permeable to the dyes, allowing their entry and acquiring a pink or red color, while those with integral membranes were not stained. For each of the four established evaluation times, an aliquot of thawed semen was taken (5 µL) and homogenously mixed on a slide with 45 µL of a coloring solution (eosin 1 % -nigrosin 5.3 % - citrate 2.9 %) for a final dilution ratio of1:10 (semen: dye) (Medina-Robles et al., 2019). This mixture was extended on a slide and 100 spermatozoa were observed through an optical microscope (magnification of 400X) to determine the percentage that maintained the integrity of the plasma membrane. Media ± SD of 3 analyzed slides was reported.
pH
This variable was determined in each of the established PST with a digital pH meter LAB 850 (Schott Instrument, Mainz, Germany).
Fertility test
Oocytes were obtained from a single female whose final maturation and spawning were induced with carp pituitary extract (CPE, Stoller Fisheries, Spirit Lake, Iowa, USA) at a total dose of 5.75 mg kg-1 wet weight, with the first application corresponding to 10 % of the total dose at zero hours and the remaining dose after 12 hours. Spawning occurred 8 h after the last dose at a temperature of 25.9 ± 0.5°C. For the controls (fresh sperm), four sexually mature males (sperm motility > 90 %, weight: 3.1 ± 0.2 kg) were injected with a single dose of CPE (4 mg kg-1 wet weight), simultaneously with the first treatment of the females. Fresh sperm was extracted at the time of spawning. For each replicate (in triplicate), 2 g of oocytes (~2800 oocytes) were weighed and fertilized with 400 µL of cryopreserved sperm from each post-thaw storage time and 100 µL of fresh sperm for the controls (Ramirez-Merlano et al., 2011). Macrotubes with the cryopreserved semen were thawed before spawning according to the storage time established for each treatment and synchronized with the projected moment of spawning. After spawning, cryopreserved semen was mixed with the oocytes and activated with 4 mL of each of the two activating solutions described previously, controls were activated with 4 mL of incubation water. Afterward, oocytes were washed with water from the recirculation system where they were finally incubated. The embryonic development was carried out in 2 L experimental incubators with a continuous flow of water (temperature: 26.9 ± 0.5°C; dissolved oxygen: 6.9 ± 0.1 mg.L-1; pH: 7.5 ± 0.04). Fertility was assessed six hours after fertilization, and the proportion of fertilized oocytes (those of translucent appearance and blastopore embryonic stage) and unfertilized oocytes (partial or total white coloration) was calculated in a sample of 100 embryos taken at random from experimental incubators using a one-centimeter glass tube as a pipette. The collected oocytes were placed in a petri dish previously marked with a grid to facilitate the differential count expressed as a percentage (Castillo-Losada et al., 2016).
Statistical Analysis
The data were described as mean ± standard error of the mean (SEM). Normality and homogeneity of variances were verified with Kolmogorov-Smirnov and Bartlett tests, respectively. Two-way analysis of variance (ANOVA) - factor 1: activating solutions (1 % NaHCO3 and 0.9 % NaCl) and factor 2: PST (0, 15, 30, and 60 min) was carried out to analyze the effects of the treatments on sperm motility, DM, and fertility. To analyze the effect of PST on pH and SMI variables, a one-way ANOVA was carried out, also verifying the assumptions of normality and homogeneity of variances. The data of variables expressed in percent (%) as sperm motility, SMI, and fertility were transformed with the root of the archsinus. The Tukey test was the posthoc test used to compare the means between the different treatments, including control concerning each treatment in the fertility test. The minimum significance level was 95 % (p < .05). The statistical analyzes were performed using R packages (ver. 4.0.1).
RESULTS
The interaction between activating solutions and PST had a significant effect on DM (p = .0398). The type of solution that activates the semen significantly affects sperm motility and fertility percentages (p < .0001), in a similar way as the PST, demonstrated to have a highly significant effect on each of the sperm variables (p < .0001). Sperm motility reduced significantly (p < .05) through the PST for both treatments activated with sodium bicarbonate (SB) and with sodium chloride (SC) (Fig. 1a). The percentage of sperm motility was significantly higher in all PST (0, 15, 30 and 60 min) activated with SC (80 ± 0.0 %, 58 ± 2.5 %, 44 ± 3.8 % and 25 ± 1.9 % respectively), compared with SB (Fig. 1a). The PST hurt sperm motility, but it did not become zero after 60 min. The DM is also reduced through the PST. The semen activated with SB did not show significant differences on DM between times 0 and 15 min, but at 30 and 60 min they were significantly lower compared to time 0 (Fig. 1b). The samples activated with SC. The treatments activated with SC did not present significant differences up to 30 min PST. Regardless of activation solution (SB or SC), PST has a significant negative effect (p < .05) on DM at 60 min (52 ± 2.6 s and 55 ± 1.8 s, respectively) compared to time 0 min (79 ± 4.4 s and 70 ± 2.7 s, respectively) (Fig. 1b). The integrity of the sperm membrane remained stable without significant differences (p > .05) through the PST until 30 min. However, it was significantly lower at 60 min PST (76 ± 1.3 %) compared to the time at 0 min (82 ± 1.2 %) (p < .05) (Fig. 2a). The pH of the semen did not vary significantly (p > .05) through the PST (Fig. 2b). Fresh sperm (control) reached a fertility rate of 97 ± 0.8 %, with no significant difference compared with cryopreserved semen activated with SB at 0 - and 15 - min PST (93 ± 1.3 % and 90 ± 3.1 %, respectively). In all other PST, the fertility rate dropped significantly compared to fresh sperm (p < .05). The semen activated with SB presented a higher fertility rate (67.0 ± 1.3%) than that activated with SC (35.0 ± 1.8 %) after 60 min (p < .05). Overall, the fertility rate was significantly affected with the use of SC as activation solution. Despite the fertility rate of semen activated with SC being significantly lower than that activated with SB (p < .05), there was no significant difference between both activation solutions at 0 and 15 min (p > .05) (Fig. 3).
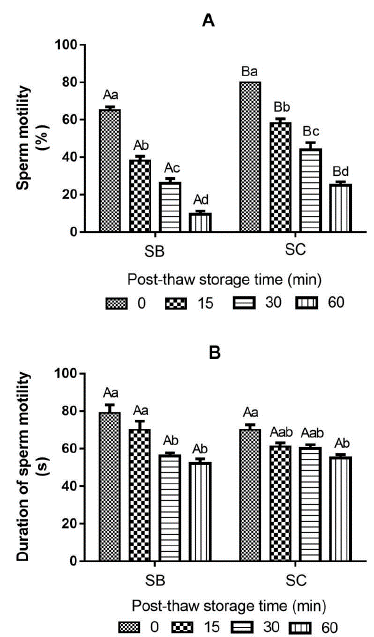
Figure 1 Sperm motility (a) and duration of sperm motility (b) of cryopreserved sperm of Cachama blanca (Piaractus orinoquensis) at different post-thaw storage times (PST). SB: 1 % sodium bicarbonate; SC: 0.9 % sodium chloride. Different capital letters indicate a significant difference between activation solutions in the same PST (p < .05). Different lowercase letters indicate a significant difference between PST for the same activation solution (p < .05). Values are shown as mean ± SEM. n = 5
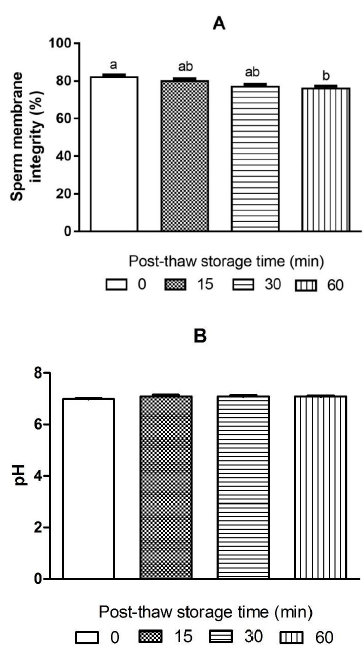
Figure 2 Sperm membrane integrity (a) and pH (b) of cryopreserved sperm of Cachama blanca (Piaractus orinoquensis) at different post-thaw storage times (PST). A - Different letters indicate a significant difference between PST (p < .05). B - There was no significant difference in pH between PST (p > .05). Values are shown as mean ± SEM. n = 5
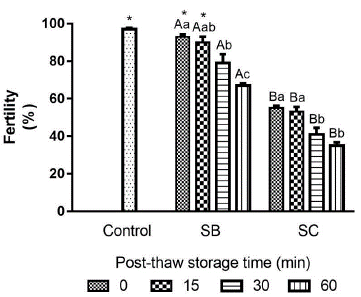
Figure 3 Fertility of long-term (seven years) cryopreserved sperm of Cachama blanca (Piaractus orinoquensis) at different post-thaw storage times (PST). SB: 1 % sodium bicarbonate; SC: 0.9 % sodium chloride. Control: fresh sperm. Different capital letters indicate a significant difference between activation solutions in the same PST (p < .05). Different lowercase letters indicate a significant difference between PST for the same activation solution (p < .05). *Treatments without significant differences from control (p > .05). Values shown as mean ± SEM. Control n = 4; SB-SC n = 5
DISCUSSION
Results showed the effectiveness of the method used for semen cryopreservation of P. orinoquensis for seven years. Post-thaw motility at time 0 was higher than those reported in Piaractus brachypomus 33 % (Ramirez-Merlano et al., 2011), 49 % (Nascimento et al., 2010), and 40 % (Navarro et al., 2004) using 10 % DMSO. On the other hand, motilities of 37 %, 57 %, and 80 % were reported using 5 % ethylene glycol, 10 % methanol, and 5 % DMSO respectively (Fresneda et al., 2004; Nascimento et al., 2010). DMSO is an intracellular cryoprotectant that causes membrane lipid and protein reorganization. During cryopreservation, DMSO improves membrane fluidity causing greater dehydration at lower temperatures and thus an increased cell ability to survive (Holt, 2000). Its concentration and equilibrium time (interaction with the cell) can be negative in sperm physiology, generating effects such as osmotic shock or biochemical problems (Hu et al., 2011). In our laboratory concentrations of10 % v/v be the most effective for different species of characiforms.
Osmolality of solutions 0.9 % SC (308 mOsm kg-1) and 1 % SB (185 mOsm kg-1) activated the sperm motility of thawed sperm at all PST, being higher at PST 0 min (80 % and 65 %, respectively). The osmolality of the seminal plasma (287.2 ± 18.3 mOsm kg-1) of P. orinoquensis without cryoprotectants was within the range reported by Suárez-Martínez et al. (2019) in the same breeding season, April (261.53 ± 19.89 mOsm kg-1) and May (307.55 ± 19.06 mOsm kg-1). Osmolality of activator solutions was able to trigger post-thawing sperm motility, being a significant influencing factor in this variable, as well as in the fertility rate. In most freshwater species, hyperosmolality of the aqueous medium is the main trigger of sperm motility, leading to changes in polarization of the membranes, possibly due to the variation in the internal concentration of K+, Ca2+ and a cAMP-independent activation system (Dumorné et al., 2018). SC solutions with lower osmolalities than those used in the present study (50-100 mOsm kg-1), have been used as activators of the motility of cryopreserved semen in other characids such as Cachama blanca P. brachypomus (Nascimento et al., 2010), Cachama negra Colossoma macropomum Cuvier (Oliveira et al., 2016), streaked prochilod Prochilodus lineatus Valenciennes (Viveiros et al., 2009), among others. Similarly, 1 % SB has been used as an activator of thawed semen in P. brachypomus (Navarro et al., 2004; Viveiros et al., 2009; Ramirez-Merlano et al., 2011). There were no significant variations in percentage motility in the fresh semen of P. brachypomus activated with hypoosmotic solutions containing SC (50 or 100 mOsm kg-1) and SB (100 or 184 mOsm kg-1), but reduced DM when the activator was SC 100 mOsm kg-1 (de Almeida et al., 2012; Moreira et al., 2012). In this study, the osmolality of SC was higher than that of fresh seminal plasma, however, it allowed for the activation of motility in thawed semen showing greater motility than in cryopreserved sperm activated with hypoosmotic SB (184 mOsm kg-1). These are unexpected results considering the knowledge and previous studies reporting that cryopreserved sperm should be activated in solutions with lower osmolality than those used for fresh sperm. These results open the possibility of an alternative mechanism of activation for sperm in P. orinoquensis. However, most studies in characiforms show that the main mechanism of activation is hypoosmolality. Viveiros et al. (2019) stated that hypoosmolality is a key factor in the process related to the activation of sperm motility. Similarly, they observed that glucose solutions free of Na+, and Ca2+ fully activated the post-thaw sperm motility of Brycon insignis Steindachner, in a similar way to those observed after activation with NaCl, sodium citrate, and KCl, concluding that the osmolality of the activating solution instead of the presence of ions is the trigger for the activation of sperm motility. Thus, it is possible that the hyperosmolar conditions generated by the extender due to the extracellular presence of large non-permeable molecules such as glucose and those derived from egg yolk, together with the presence of DMSO, result in an osmolality that allows activation with substances such as SC (308 mOsm kg-1) that have a similar or slightly higher osmolality than that measured in fresh undiluted semen.
The PST reduced the spermatozoon motility of P. orinoquensis in all evaluated times up to 55 % at 60 min compared to time 0 min and independently of the activation medium used. These results agree with Aramli & Nazari (2014), who reported a 47 % decrease in the sperm motility of the Persian sturgeon Acipenser persicus 60 min after thawing. Viveiros et al. (2019) observed a significant reduction in the activation capacity of Brycon insignis sperm after 20-25 min post-thaw. They have also observed it in the salmon river Brycon orbignyanus Valenciennes and dorado Salminus brasiliensis Cuvier, just a few seconds after thawing. Alterations in activation processes can be related a sperm membrane damage (adenylate cyclase, ion channels, clustering of other proteins) that can affect the signaling pathway for sperm activation. Furthermore, ATP degradation, fragmentation of nuclear and mitochondrial DNA (genome), degradation of kinases and other cytosolic proteins (proteome) are some molecular factors that can be affected during cryopreservation and reduce the fertilizing capacity and motility of sperm in fish (Martínez & Pardo-Carrasco, 2010). In Caspian trout Salmo trutta caspius (Golshahi et al., 2015), brook trout Salvelinus fontinalis (Horváth et al., 2015) and brown trout Salmo truta m. fario (Nynca et al., 2015), no significant effect of PST (0 and 60 min) on motility was observed. In the same way, Bernáth et al. (2017) did not record significant statistical differences in sperm motility between time 0 and 120 min in different phenotypes of goldfish Carassius auratus Linnaeus. Thawed spermatozoa of streaked prochilod Prochilodus lineatus were shown to be very resistant and motility can be achieved up to 40-50 min after thawing without loss of sperm capacity of being activated (Viveiros et al., 2016). The present study is the first report of fertility in tropical freshwater fish using cryopreserved sperm after 7 years of storage. The fertilization rate with cryopreserved semen of P. orinoquensis decreased progressively and significantly with the increase in PST, similar to that reported in Persian sturgeon, in which fertilization and hatching rates decreased significantly after 60 min of PST (Aramli and Nazari, 2014). In contrast, Horváth et al. (2015) found that fertility rate and hatching percentage in salmonids were maintained regardless of the PST used. The current study shows an independent effect of the PST and the activating solution on fertility rate, however, interactions of these two factors did not have a significant effect on it. Although the purpose of the cryopreservation process is to preserve the viability of sperm cells during the long term, a reduction in the fertility rate of sperm was observed in most PSTs compared to the control (fresh sperm). Chen et al. (2010) found differences in DM, sperm motility, and fertilization percentage in red seabream Pagrus major sperm cryopreserved for 1, 13, 26, 48, and, 73 months, the trend showed a continuous decline in duration and longevity between months 1 and 73 months, especially after month 26. Similarly, in the silver barb Barbodes gonionotus, sperm motility was significantly reduced in thawed semen 10 months after cryo-storage (Vuthiphandchai et al., 2015). Viveiros et al. (2019) evaluated B. insignis semen cryopreserved for five years, reporting post-thaw motility above 70 % through the use of different activator solutions with a wide range of osmolalities (0-200 mOsm kg-1), including SB and SC, without finding significant differences in the post-thaw motility obtained by the use of these two substances.
PST significantly affected sperm integrity after 60 min. The progressive reduction in sperm quality during PST can be attributed to instability caused by alterations in the cell membranes because of freezing and thawing processes (Viveiros et al., 2017, 2019). Cryopreservation causes loss of membrane fluidity due to the rigidity of the lipids during cooling in addition to the denaturation of the proteins, resulting in disruption of transmembrane transport and ion exchange. In addition, it generates ruptures that affect the conformation of the cytoskeleton, reducing the ability to the union between the sperm and the oocyte in the thawed semen (Karow, 2001). Moreover, the cryogenic effect can damage membranes of organelles such as mitochondria, affecting the availability of ATP for sperm movement (Figueroa et al., 2019), a situation that would partially explain the impaired motility and reduced fertilization capacity during PST. In all PSTs, the pH tended to be neutral (7.0), being a favorable characteristic for seminal quality after defrosting. Although sperm motility and fertility were significantly reduced by PST, fertilizing capacity remained at acceptable values after 60 min when sperm motility was activated with SB 1 %. SC 0.9 % allowed the activation of sperm motility of cryopreserved semen; however, fertility tests were not satisfactory as those obtained with SB.
CONCLUSIONS
The fertility rate with semen cryopreserved for 7 years and kept up to 15 min PST, was statistically equal to that determined with fresh semen. Long-term semen storage in P. orinoquensis is viable and potentiates its use for conservation programs, artificial reproduction, and genetic improvement.















