INTRODUCTION
Pathogenic fungi are responsible for 70 to 80% of diseases found in plants, representing one of the main problems affecting the yield and quality of crops and the post-harvest life of fresh fruits and vegetables (Saharan et al., 2015; Li et al., 2017; Basu et al., 2021). Chemical fungicides have been used excessively to control the spread of phytopathogenic fungi; however, the application of these agrochemicals is expensive and may pose risks to human health and the environment (Aslam et al., 2020; Sarfraz et al., 2018). In addition, continuous use of the same fungicide can increase the potential risk of developing fungicide-resistant strains (He et al., 2019). Therefore, new alternatives have been sought in recent years for controlling phytopathogenic fungi, such as the use of plant extracts containing phenolic compounds, which are considered a natural alternative to conventional fungicides (Filippi et al., 2020). These phenolic compounds play a key role in the adaptation of plants against biotic and abiotic stress, especially in the defense against fungi and insects (Lattanzio et al., 2006). The compounds are synthesized before or during infection, providing a natural chemical shield, and acting as a toxin against the invading pathogen (Maurya et al., 2007; Roy et al., 2017; Schöneberg et al., 2018). The plant responds to the attack of phytopathogenic microorganisms by generating apoplastic reactive oxygen species (ROS) as an oxidative burst to prevent the progression of the pathogen toward the conductive vessels. In addition, phenolic acids and flavonoids are capable of inhibiting and suppressing the germination of conidia, reducing mycelial growth, and deforming hyphae (Filippi et al., 2020; Batnini et al., 2021). It has been reported that phenolic acids such as trans-Cinnamic acid, ferulic acid, and p-Coumaric acid can inhibit the growth of phytopathogenic fungi of the genus Colletotrichum spp. (Roy et al., 2017). Ferulic acid is an excellent growth inhibitor of Sclerotium rolfsii Sacc. (Maurya et al., 2007). Vanillic acid, p-Coumaric acid, and salicylic acid have antifungal efficacy against the genera Fusarium, Aspergillus, and Penicillium (Zabka and Pavela, 2013). Moreover, some flavonoids, such as naringenin, quercetin, and resveratrol, are synthesized in higher concentrations in beans in the presence of the pathogen Rhizoctonia solani (Mayo-Prieto et al., 2019). However, the recovery of these bioactive compounds depends on the plant species, plant structures (stem, leaves, flowers, and roots), methodology, and type of solvent extraction (polar, intermediate polarity, and non-polar solvents or mixtures of these), which leads to differences in antioxidant and antifungal activity of the extracts (Sultana et al., 2009; Ayala-Zavala et al., 2012; Andrade-Andrade et al., 2018; Fernández-Rodríguez and Ruiz-López, 2021). Regarding the type of plant, it has been observed that higher concentrations of phenolic compounds with antioxidant activity are synthesized by the wild species growing in agricultural fields than by the main crops (Barrientos-Ramírez et al., 2019).
Tinantia erecta is a native species of Latin America that belongs to the Commelinaceae family and that can be found as a wild plant mainly in crop fields of corn, coffee, and beans. It is used as forage, as an edible wild plant, and as an ornament (Villaseñor and Espinosa, 1998; Mera-Ovando et al., 2003; Marciniec et al., 2019). Due to its high adaptability and distribution, this species could serve as a source of compounds with potential antifungal activity. However, there are no reports on the concentration and distribution of phenolic compounds in the various organs of T. erecta, nor on the most effective solvent for extracting the highest concentration of these antioxidant compounds. Therefore, the present research was undertaken to evaluate the antioxidant activity and quantify the phenolic compounds and flavonoids present in ethanolic (70% v/v) and aqueous extracts of T. erecta stems, leaves, and roots, and to evaluate their antifungal activity against phytopathogenic fungi.
MATERIALS AND METHODS
Plant material
Tinantia erecta is a wild herbaceous plant that was collected at Zitacuaro, Michoacan, Mexico (19°25' N, and 100°22' W, 1920 m.a.s.l) in August 2019. According to the Köppen climate classification system, the climate is classified as a subtropical highland climate (García, 2004). The plant was identified, with an identification number of ADH-F 15. The plants were shade-dried for 2 weeks, after which the samples were cut and stored at room temperature before further processing.
Biological material
The plant pathogens evaluated included the oomycete Phytophthoracapsici, and the fungi Sclerotiumrolfsii, Colletotrichum gloeosporioides, Fusarium oxysporum, and Rhizoctonia solani, all of which were obtained from the collection of microbial cultures of the Colegio de Postgraduados, Campus Montecillos (Mexico).
Preparation of plant extracts
For each organ (leaves, stems, and roots), 100 g of sample was put into a 2-L glass container. To obtain the ethanolic extracts, 1000 mL of 70% ethanol was added until the solvent reached the total volume; afterward, the samples were stored in the dark for 15 days at 20 °C. The same process was carried out to obtain the aqueous extracts.
The ethanolic extracts were vacuum evaporated in a rotary evaporator (BUCHI, Model R100, Flawil, Switzerland) for 2 h until a dried sample was obtained. Then, the samples were stored at 4 °C under dark conditions. Aqueous extracts were freeze-dried (Labconco, Model 79480, Kansas City, MO, USA) for 72 h at -40 °C.
Total phenol and flavonoid content
The determination of the total phenolic content of the extracts was carried out according to Singleton and Rossi (1965), with slight modifications. Briefly, 25 mg of extract was mixed with 10 mL of 70% ethanol. The mixture was then sonicated (Ultrasonic Cleaner, Mod. 32V118A, Freeport, IL, USA) at 40 kHz for 10 min at 25 °C. Later, the sample was centrifuged (Thermo Scientific, Mod. ST 16R, Waltham, MA, USA) at 10,000 xg for 10 min at 4 °C. The supernatant was recovered, and a sample was used and mixed with Folin-Ciocalteu reagent, sodium carbonate, and distilled water. The mixture was incubated for 60 min in darkness, and absorbance was later measured at 725 nm in a spectrophotometer (Spectrophotometer model 6715 UV/Vis, Jenway, Techne Inc., Staffordshire, UK). The total content of flavonoids in the extracts was determined according to Zhishen et al. (1999). The absorbance was measured at 415 nm. The results of the total phenol and flavonoid contents were expressed as mg of gallic acid equivalents (mg GAE) and mg of quercetin equivalents (mg QE) per gram of extract, respectively.
Determination of phenolic compounds
Phenolic acid and flavonoid contents were determined according to Aguiñiga-Sánchez et al. (2017). The content and type of analytes in the extract from each sample were identified by high-performance liquid chromatography with diode array detection (HPLC-DAD, Agilent Technologies 1100, EUA). The quantification of phenolic acids was performed using a Nucleosil column/Macherey-Nagel (125 x 4.0 mm) with a gradient of (A) H2O, pH of 2.5, with TFA (trifluoroacetic acid), and (B) ACN (acetonitrile). Additional experimental parameters measured during the analysis were: flow, 1 mL min-1; temperature, 30 °C; varying injection volume; and analysis duration of 25 min. The acids used as standards were: caffeic, gallic, chlorogenic, vanillic, p-hydroxybenzoic, p-Coumaric, ferulic, and syringic. On the other hand, the flavonoids were quantified using a column Hypersil ODS/Hewlett Packard (125 x 40 mm), with a gradient of (A) H2O, pH of 2.5, with TFA (trifluoroacetic acid), and (B) ACN (acetonitrile), and according to the following parameters: flow, 1 mL min-1; temperature, 30 °C; varying injection volume; and analysis duration of 25 min. The standards used were: rutin, phloridzin, myricetin, quercetin, naringenin, apigenin, phloretin, and galangin. The results were expressed as µg g-1 of extract.
DPPH free radical scavenging assay
The determination of antioxidant activity by DPPH assay was carried out according to Brand-Williams et al. (1995). Briefly, an aliquot was obtained of the supernatant, which was used to measure total phenols. Then, the sample was mixed with the DPPH-+ radical (6 x 10-5 M), after which the mix was incubated at 4 °C for 60 min and the absorbance was measured at 517 nm. The results were expressed as mM Trolox equivalents (TE) per gram of extract.
Trolox equivalent antioxidant capacity method (TEAC)
The TEAC assay was carried out according to Re et al. (1999), using ABTS (7 mM) and potassium persulfate (2.45 mM) in a ratio of 1:1 to form the ABTS-+ radical. Samples were incubated in darkness for 16 h, after which the supernatant of each extract was mixed with ABTS-+ radical. Later, the mixtures were incubated in a dark chamber for 6 min. Finally, the absorbance was measured at 734 nm. The results were expressed as mM Trolox equivalents (TE) per gram of extract.
Evaluation of antifungal/anti-oomycete activity
The antimicrobial activity of extracts was determined according to Gutiérrez-Tlahque et al. (2019). Each independent extract was mixed with PDA (Potato Dextrose Agar) at a concentration of 250 µg mL-1. A 5-mm-diameter disc with the growth of each pathogenic microorganism was placed on the PDA plates (60 x 15 mm) and incubated at 28 °C for 8 days in an incubator (Felisa, Model FE-133D, Zapopan, Mexico). The percentage of radial growth inhibition was calculated after 7 days of incubation, based on the diameter of the microorganism colonies measured in the treatment and the control plates. Plates with 3.9% PDA and 3.9% PDA plus 0.5% (v/v) acetone were used as negative controls, and plates with the fungicide Captan (250 µg mL-1) were used as a positive control.
The following formula was used to determine the percent inhibition:
% inhibition = (A- B) / A
A = Diameter of the fungus/oomycete colony on negative control plates
B = Diameter of the fungus/oomycete colony on treatment plates (PDA plus extracts) or positive control plates (PDA plus Captan).
Statistical analysis
A two-factor factorial design was used. The variation factors were the extraction solvent (70% water and 70% ethanol) and the plant organ (leaf, root, and stem). The data collected were subjected to analysis of variance (ANOVA), and the means among treatments were compared by Tukey's test (p < 0.05) for each variable analyzed. All analyses were carried out using SAS System for Windows v9.4.
RESULTS AND DISCUSSION
Antioxidant activity
The Fig. 1 shows the effect of the extraction solvent of phenolic compounds and their antioxidant activity. It is observed that the concentration of total phenols (TPC) (8.1 mg GAE g-1) and flavonoids (TFC) (19.9 mg QE g-1) extracted with 70% aqueous ethanol were 1.6 and 6.7 times higher than that extracted with water. According to the literature, the combination of polar organic solvents with water facilitates the extraction and yield of various chemical compounds such as polyphenols, whereas in pure water there is a greater solubility of carbohydrates and terpenes (Diem et al., 2013). Polyphenols are particularly better extracted in more polar solvents, mainly in aqueous ethanol, compared to other aqueous solvents such as aqueous methanol and aqueous acetone (Sultana et al., 2009).
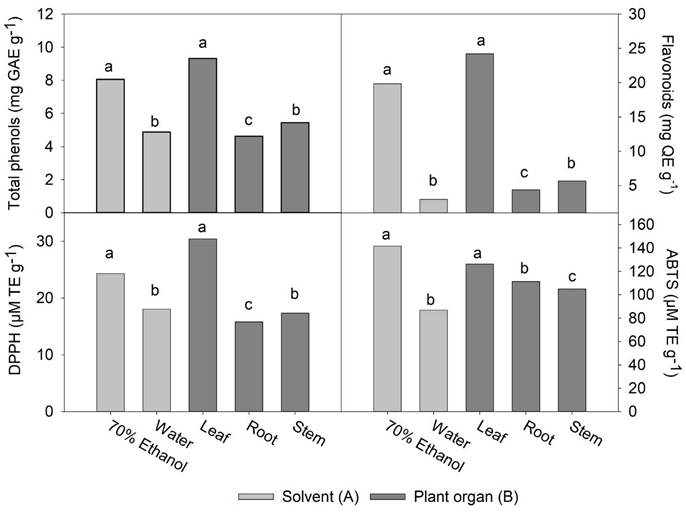
Figure 1 Effect of different extraction solvents (70% ethanol and water) (A) and different plant organs (leaf, root, and steam) (B) on the content of total phenols, flavonoids, and on the antioxidant activity of Tinantia erecta. Means with a different letter in the same factor (A or B) indicate a statistically significant difference (Tukey, P < 0.05).
On the other hand, a greater extraction power of 70% aqueous ethanol in TFC (6.7 folds) compared to TPC (1.6 folds) may be in response to the fact that flavonoids are compounds of high molecular weight and with a greater number of phenol groups; therefore, they are more soluble in polar organic compounds than in water (Diem et al., 2013; Meneses et al., 2013).
The antioxidant activity of the 70% aqueous ethanol extract, as evaluated by the inhibition assays of the DPPH (24.3 µM TE g-1) and ABTS radicals (141.5 µM TE g-1), was significantly higher than in the aqueous extracts, with values of 18.0 and 86.9 µM TE g-1, respectively. The difference is related to a higher concentration of phenols and total flavonoids in the 70 % aqueous ethanol extracts (Fig. 1).
Our results agree with previous studies in which the effects of the solvent in extracting phenolic compounds from different types of plants were evaluated, and greater antioxidant activity was observed at a higher concentration of phenolic compounds (Ayala-Zavala et al., 2012; Meneses et al., 2013; Ferhat et al., 2017). In our study, we observed that the antioxidant activity reported by the ABTS assay was too high relative to the antioxidant activity reported by the DPPH assay. This discrepancy may be due to the high concentration of total flavonoids in the extracts. Since flavonoids are compounds of high molecular weight, there could be less accessibility to the site of the radical DPPH for its respective reduction, and therefore less antioxidant activity is expressed in the extracts (San Miguel-Chávez, 2017).
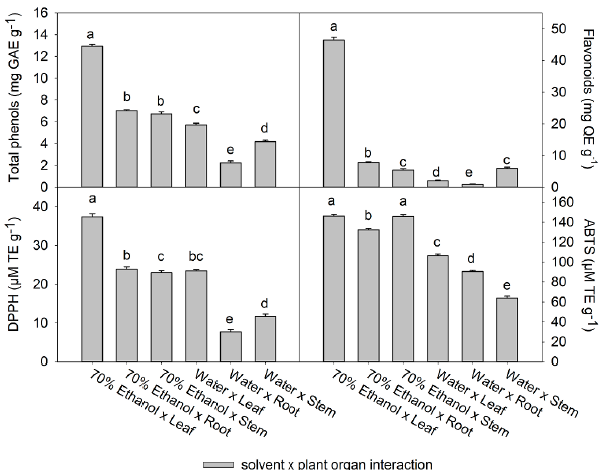
Figure 2 Effect of the extraction solvent x plant organ interaction on the content of total phenols, flavonoids, and on the antioxidant activity of Tinantia erecta. Bars are mean value ± standard error (n= 3). Means with a different letter indicate a statistically significant difference (Tukey, P < 0.05).
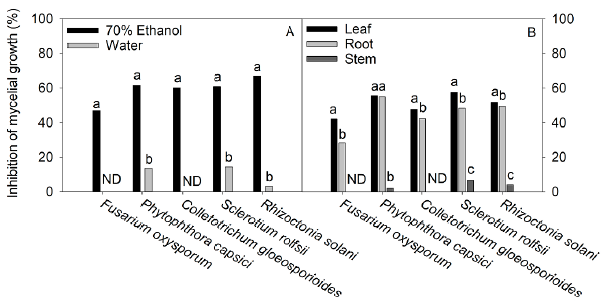
Figure 3 Effect of different extraction solvents (70% ethanol and water) (A) and different plant organs (leaf, root, and steam) (B) of Tinantia erecta on inhibition in the mycelium growth of F. oxysporum, P. capsici, C. gloeosporioides, S. rolfsii and R. solani. Means with a different letter in the same pathogen assessed indicate a statistically significant difference (Tukey, P < 0.05). ND: not detected.
The content of total phenols and flavonoids in different organs of T. erecta is displayed in Fig. 1. The content of total phenols in the leaves was the highest (9.3 mg GAE g-1), while the lowest concentration was found in the roots (4.6 mg GAE g-1). A similar trend was observed when comparing the content of total flavonoids: values of 24.2, 5.6, and 4.4 mg QE g-1 were found for leaf, stem, and root extracts, respectively. It has been reported that in other wild plants, the content of phenolic compounds is higher in leaves than in other plant organs (Andrade-Andrade et al., 2018; Muthukrishnan et al., 2018). The concentration of these compounds is similar to that found in other edible wild species of the genera Amaranthus, Chenopodium, and Portulaca (Santiago-Saenz et al., 2018, 2020). It may be due to the highest metabolic activity of the leaves, in addition to greater exposure to light and attack by pathogens and/ or herbivores (Andrade-Andrade et al., 2018). Also, in the leaf, high antioxidant activity in vitro was observed in both assays, with values of 30.4 µM TE g-1 for the DPPH assay, and 126.3 µM TE g-1 for ABTS. Even though the rest of the plant organs did not present high antioxidant activity, the bioactive compounds identified are relevant in the area of human nutrition.
The Fig. 2 shows the effect of the interaction between the extraction solvent and the plant organs used. The highest content of phenols (12.9 mg GAE g-1) and total flavonoids (46.4 mg GAE g-1), and the highest antioxidant activity (146.3 µM TE g-1) was found in the 70% ethanol x leaf interaction, followed by the 70 % ethanol x root interaction. The effect of the plant organ on the compound's content and the antioxidant activity is also presented in Fig. 1. The lowest concentration of phenols, flavonoids, and antioxidant activity by the DPPH method was found in roots; however, in the interaction with the solvent (70 % ethanol), the content of these compounds increased per each gram of extract obtained. On the other hand, the different interactions of the water solvent with the various organs of the plant presented the lowest values of the variables evaluated (Table 2). The values in the water x leaf interaction were among the highest in the water x plant organ interactions, with values of 5.7 mg GAE g-1 for phenols, 2.1 mg QE g-1 for flavonoids, and antioxidant activity of 7.7 and 90.5 jM TE g-1 by DPPH and ABTS, respectively.
According to the results obtained for total phenol and flavonoid content, the concentration of flavonoids quantified by HPLC of T. erecta is higher than the concentration of phenolic acids (Tables 1 and 2). The most important flavonoids identified were phloridzin, naringenin, and rutin. On the other hand, among the phenolic acids, high concentrations of ferulic acid, vanillic acid, and p-hydroxybenzoic acid were found. Ferulic acid, vanillic acid, and p-hydroxybenzoic acid have been reported to have antioxidant, anti-inflammatory, antimicrobial, and antidiabetic properties (Manuja et al., 2013; Patzke and Schieber, 2018; Kaur et al., 2022). The quantification of the content ofphenolic compounds using HPLC showed that the most effective extraction solvent was 70% ethanol (Table 1). For example, the content of phenolic compounds obtained from leaves using 70% ethanol was 81.8 mg per g of extract, whereas the concentration obtained using water was only 6.6 mg per g of extract. The results of this study indicate that ethanol facilitates the extraction of phenolic compounds, especially from the leaves of T. erecta. Many types of plants synthesize a large variety of phenolic compounds, which are biologically active metabolites that act as a defense mechanism against the attack of plant pathogens; being toxic to these invading organisms (Lattanzio et al., 2006; Roy et al., 2017; Schöneberg et al., 2018). In addition to the phenolic compounds, T. erecta extracts are also a good source of flavonoids, such as naringenin, phloridzin, and rutin (Table 2). In addition to antifungal and antimicrobial properties, flavonoids have other important properties such as antidiabetic, antioxidant, anti-inflammatory, antitumor, and anticancer properties (Prasad & Prasad, 2019; Tian, et al., 2021). It has also been reported that naringenin has antiatherogenic, antidepressant, immunomodulatory, DNAprotective, lipid-lowering, and activation of peroxisome proliferator-activated receptor effects, and helps improve memory (Venkateswara et al., 2017).
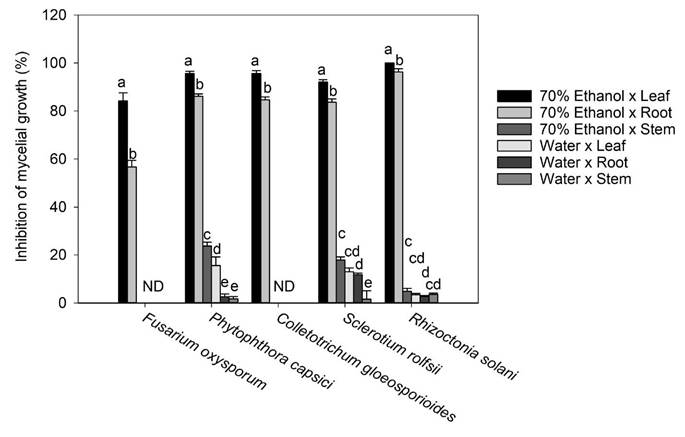
Figure 4 Effect of the extraction solvent x plant organ interaction on inhibition in the mycelium growth of F. oxysporum, P. capsici, C. gloeosporioides, S. rolfsii, and R.a solani. Bars are mean value ± standard error (n= 3). Means with a different letter in the same pathogen assessed indicate a statistically significant difference (Tukey, P < 0.05). ND: not detected.
Antifungal/anti-oomycete activity
The results presented in Fig. 3 showed that the inhibition in the mycelium growth of the pathogens assessed was much greater when the extracts obtained with 70% ethanol were used than when the aqueous extracts were tested. The order of sensitivity of phytopathogenic fungi using 70% ethanol extracts was as follows: R. solani (67%) > P. capsici (61.4%) > S. rolfsii (60.8%) > C. gloeosporioides (60.1%) > F. oxysporum (47%). With the aqueous extracts, the order was as follows: S. rolfsii (14.2%) > P. capsici (13.6%) > R. solani (3.2%). According to our findings (Fig. 1, Table 1, and Table 2), the 70% ethanolic extracts present a higher content of flavonoids and phenolic acids than the aqueous extracts; thus, these compounds could be responsible for the inhibition of mycelial growth of R. solani and S. rolfsii. According to Mayo-Prieto et al. (2019), in bean plants (Phaseolus vulgaris L.) an increase in flavonoids has been detected in plants infected with R. solani. In sorghum, a high content of phenolic acids-ferulic acid in particular- is synthesized to combat the S. rolfsii attack (Maurya et al., 2007). It should also be noted that the water extracts have a low concentration of polyphenols and therefore could be less efficient in inhibiting the growth of these microorganisms.
Table 1 The phenolic acid content in extracts obtained from the solvent extraction * plant organ interaction (Tinantia erecta)
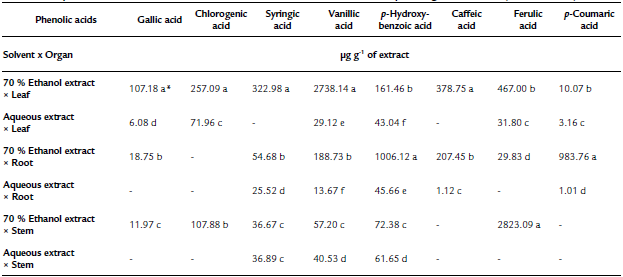
*Means with a different letter in the same column indicate a statistically significant difference (Tukey, P < 0.05).
Regarding the activity of the extracts from the different plant organs against phytopathogenic microorganisms (Fig. 3), our results indicate that the leaf extracts exhibited strong antifungal activity against the tested pathogens, followed by the root extracts, while stem extracts were the least effective.
Our study showed that the antifungal power of the different plant organs has a direct relationship with the content of phenolic compounds and with their antioxidant capacity (Fig. 1). The pathogenic fungus S. rolfsii was the most sensitive to the antifungal effect of the extracts from the various plant organs, with a 57.5% inhibition of fungal growth; whereas F. oxysporum was the most resistant pathogen, followed by C. gloeosporioides. The resistance of F. oxysporum against extracts of T. erecta (highest inhibition: 42.1%) may be due to a higher concentration of vanillic acid, p-hydroxybenzoic, quercetin, and rutin about ferulic acid, which has been shown to have an excellent antifungal effect against the pathogens of the genus Fusarium, while the remaining phenolic acids slightly stimulate the growth of this fungus (Schöneberg et al., 2018). However, the presence of vanillic acid and p-hydroxybenzoic acid in the extracts of T. erecta could have a fungistatic effect against other organisms that are sensitive to these flavonoids, such as Alternaria alternata (Romero-Cortes et al., 2019). Likewise, the presence of naringenin in T. erecta extracts could have an antifungal effect against Pyricularia oryzae and Gibberella fujikuroi, as well as antibacterial activity against Burkholderia glumae, Xanthomonas oryzae, and Acidovorax avenae, which are microbial rice pathogens sensitive to naringenin (Murata et al., 2020).
Table 2 Flavonoid content in extracts obtained from the solvent extraction * plant organ interaction (Tinantia erecta)
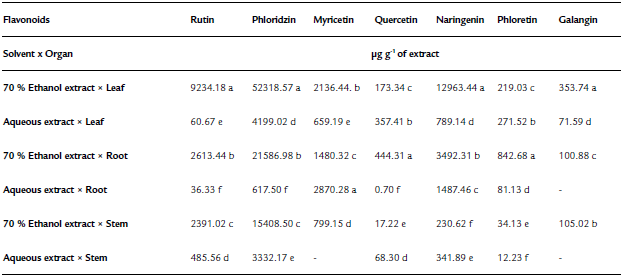
*Means with a different letter in the same column indicate a statistically significant difference (Tukey, P ≤ 0.05).
Regarding C. gloeosporioides, the literature mentions that it is sensitive to ferulic, p-Coumaric, cinnamic acids, vanillic, and caffeic acids (Roy et al., 2017; Osondu et al., 2022). However, ferulic, p-Coumaric, vanillic, and caffeic acids were found in minimal concentrations or absent in the different aqueous extracts and in the 70% ethanol extract x stem of T. erecta, which could explain why inhibition of the mycelial growth of this pathogen was not observed. On the other hand, the highest inhibitory effect (100%) at an experimental concentration of 250 µg mL-1 was observed in the 70% ethanol x leaf interaction on R. solani, more than 95% of the inhibitory effect on P. capsici and C. gloeosporioides, and more than 92 y 84% of mycelial inhibition on S. rolfsiiand F. oxysporum, respectively (Fig. 4). According to the literature, in methanolic extracts of banana peel from Musa paradisiaca L., an inhibition of up to 94.1% in the growth of R. solani has been reported, which is largely attributed to the presence of ellagic acid, gallic acid, myricetin, naringenin, and rutin (Behiry et al., 2019). Moreover, Joaquín-Ramos et al. (2020), tested the inhibitory effect of the ethanolic extracts of the leaves of Barkleyanthus salicifolius using the same panel of phytopathogenic microorganisms and they reported a lower inhibition in mycelial growth compared to our results. The toxicity of T. erecta to humans remains speculative, as no studies to date have confirmed a toxic effect; however, this wild plant has shown good properties against the plant pathogens tested at the concentration of extract evaluated.
CONCLUSIONS
This research about T. erecta revealed that the plant contains a considerable amount of phenolic compounds with antioxidant and antifungal activity. The 70% ethanol solvent was the most effective at extracting the compounds with antioxidant activity. The concentration of these bioactive compounds usually varied between plant organs; among the organs, the leaf presented the highest concentration of phenols and flavonoids. The extract with the best antioxidant properties was obtained from the 70% ethanol x leaf interaction, which also showed the best antifungal/anti-oomycete properties. A concentration of 250 µg mL-1 completely inhibited the growth of R. solani, while F. oxysporum was the least sensitive to the extract. Our results suggest that T. erecta species are an alternative for the formulation of bio-fungicides. In addition, other bioactivities of this species may be found in the future that has potential applications in food fortification and the production of new medicines.















