Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.31 no.1 Bogotá Jan./Apr. 2011
Evaluating protection systems against marine corrosion of aeronautic alloy Alclad 2024-T3
W. Aperador1, A. F. Escobar2, F. Pérez3
1 Ph.D., in Materials Engineering- Universidad del Valle. M.Sc., in Metallurgy and Materials Science-Universidad Pedagógica y Tecnológica de Colombia. Linked to Mechatronics Engineering Career, Universidad Militar Nueva Granada, Bogotá.D.C, Colombia. g.ing.materiales@gmail.com
2 Materials Engineer, Universidad del Valle, Ciudadela Universitaria Meléndez. School of Materials Universidad del Valle, Colombia. andresescobar_71@hotmail.com
3 Materials Engineer, Universidad del Valle, Ciudadela Universitaria Meléndez. School of Materials Universidad del Valle, Colombia. fapo3000@hotmail.com
ABSTRACT
This paper shows how two coating systems were obtained as an alternative for protection against corrosion of al clad 2024-T3 which is used in battery compartment manufacture for T-41 aircraft. Such systems consist of three types of organic resin: a first layer of P-115 polyester resin as the first coating on both systems, and a second layer of Hetron 197-3 polyester resin in the first system and vinylester resin in the second one. Scanning electron microscopy (SEM) was used for surface morphology analysis, showing the roughness produced by surface treatment. The coatings were electrochemically characterised by electro chemical impedance spectroscopy (EIS) and Tafel polarization curves; it was found that both systems had good performance against corrosion in a marine environment and the chemical surface preparation system had a superior protective pattern for Alodine5700 + 197-3 Hetron, a 1.42x10-12mpycorrosion rate being obtained while substratum rate was 1.59x10-7 mpy.
Keywords: Alclad 2024-T3, marine corrosion, organic coating, SEM, electrochemical impedance spectroscopy, corrosion.
Received: November 30th 2009. Accepted: January 24th 2011
Introduction
Lightweight aluminium alloys have many applications in aviation due to their great strength and low specific weight. They are therefore used in constructing aircraft fuselage and structural components, mainly 2xxx series alloys and duralumin, alloy AA2024 having greater importance as it can be plated, heattreated and is used in all kinds of coatings, structural components and fittings [Oñate, 1991; ASM Handbook; Airframe Handbook]. However, aluminium alloys are susceptible to corrosion and suffer destructive attack by chemical or electrochemical reaction in highly corrosive marine and acidifying surroundings. Thus, in addition to the problems arising from decreased mechanical properties and adding costs involved in means of inspection, control and maintenance, systems must be developed which provide practical, industrial-level corrosion protection, such as organic protection coating isolating a metal surface and creating a barrier against reactions which may occur with such environment to counteract and thus reduce high corrosion rates (ESTCP report; Bierwagen, 2001; Buchheit , 2003; Atta A, M and Nassar 2007).
Organic synthetic resin-based coatings provide excellent protection against corrosion, as long as they have been well cleaned and their surface has been prepared, followed by appropriate pre-treatment depending on the type of substrate to be protected (Atta A,M and Elsaeed, 2006; Atta A,M and Elsaeed, 2007; Sathiyanarayanan,2008). Such protection schemes can be applied in a single layer or multilayer, as in the case of coatings for the aerospace industry which generally consist of pretreatment and primary and finishing coats. Several studies related to innovation and optimising new alternative protective coatings against corrosion have been developed for application to aircraft (Reynolds, 1997 and Z.H. Fang, 2011). Research has focused on alternative coatings to traditional surface treatment (pre-treatment), this being the most representative case, and coatings provided by chemical oxidation from zirconium and titanium oxides (Sanchez-Amaya, 2007; Osborne,2001; Neuder, 2003), resins and primers without any incorporation of pigment combatants as corrosion inhibitors (Twite,1998).
The aim of this study was to evaluate and compare the corrosion pattern of several systems for alternative synthetic resin-based protective coatings compared to that used in the military aviation industry in al clad aluminium substrate alloy 2024-T3, by exposure in a 3.5 NaCl solution.
Experimental setup
T41 aircraft fuselage coatings and internal corrosion-prone areas, such as battery compartments, are made of sheet aluminium alloy 2024-T3(chemical composition shown in Table 1),1230- plated alloy, according to American Society for Testing and Materials (ASTM) B209 international specifications (Table 2) and corrosion protection coatings, a paint base according to military aviation standards (surface pre-treatment, primer and finish paint). This protection system consists of a surface treatment (trade name Alodine 5700)producing a chrome-free conversion coating in a chemical solution consisting of an 80% inorganic component including titanium, zirconium and silicon oxy fluoride species (H2 F6 + Zr Ti O (OH) (CO3) 0.5 + SiO2) and 20% organic poly-4-vinyl-phenol and amines, thereby promoting a protective coating by inorganic conversion from an organ-metallic zirconate complex and a good bonding surface for subsequent organic coating. There is also an epoxy chromatin-based surface primer, its main component being epoxy resin diluted as strontium chromate pigments for corrosion protection. A paint finish (polyurethane) is applied on top (Twite,1998).


The two synthetic resin-based protection systems involved the following implementation stages. There were two types in the case of pre-treatment; the first was chemical initiating cleaning and degreasing process with a phosphoric acid-based detergent acid at 46º C for 10 minutes, etching then being made with NaOH at 50 g / l concentration dissolved in distilled water for 1 minute at 52°C and finally anchor treatment (Alodine 5700). The other system was mechanical, specimens being polished with 200 mm SiC aasive paper and then subjected to the same process described in chemical treatment prior to Alodine 5700application.
A thin polyester resin layer (50 micron Poly-sec 115) was applied by casting for both types of preparation; the coating was homogenised over the entire application area. The resin had a 20- minutecuring time. A layer of resin composite coating antacid (197-3 Hetron polyester resin or vinyl ester resin F-010) was then applied to each pre-treatment system incorporated as filler in 20% glass flake (w / w) and 2% (w / w) catalyst ratio. A combination of pre-treatment and Hetron 197-3polyester resin was used for the electrochemical tests and named as follows: AH1 system, Alodine 5700 + Hetron 197-3 (1 layer); AH2, Alodine 5700 + Hetron 197-3 (2 layers); H1, + Hetron 197-3 mechanical polishing (one layer); H2, + Hetron 197-3 mechanical polishing (2 layers). Systems obtained for Vipel F-010vinyl-ester resin were: F1 system, mechanically polished Vipel F010 + (1 layer); F2, F010 Vipel + mechanical polishing (2 layers); AF1,Alodine 5700 + Vipel F010 (1 layer);AF2,VipelAlodine 5700 + F010 (2 layers).These systems were compared to a commonly used coating, which is a combination of Alodine 5700, Epoxy primer and polyurethane paint; this coating was called PIM (Figure 1).

The specimens were subjected to ultrasonic cleaning in an acetone bath and dried before testing. Measurements were taken with an Elcometer300thicknessgauge. Electrochemical characterization was performed on a computer (GamryPCI-4) using Tafel polarization curves and electrochemical impedance spectroscopy at room temperature. A cell consisting of a typical 3-electrode arrangement was used with working electrode having a1 cm2 exposed area, an Ag / Ag Cl reference electrode as counter electrode and a platinum wire in a 3.5% wtNaClsolution.Tafel polarization curve measurements were obtained at 0.125 mV / s scan rate in a -200 mV to +1200 mV voltage range cf open circuit potential (Ecorr). Nyquistplots were obtained by frequency sweeps in the 0.001 Hz to 100 KHzrange using 10 mV sine signal amplitude. The coatings´ chemical composition was analysed by using a high-resolution electron scanning microscope (JEOL JSM - 6490LV) equipped with a light sensitive element (EDX system) at1 nm at 30 kV resolution.
Results
Morphology and chemical composition of Alodine5700 conversion coating (SEM / EDS)
The microstructure obtained by scanning electron microscopy (Figure 2), together with EDS chemical analysis (Table 3), confirmed conversion coating formation on Alclad substrate surface (aluminium alloy 1230). The surface morphology observed by SEM (Figure 3) clearly illustrated the roughness produced by chemical oxidation and the characteristic values recorded EDS chemical composition for elements such as Zr, Ti, Al, F and O, following treatment with Alodine 5700.The 35% composition in C verified the presence of an additional organic binder promoting chemical affinity with the organic coatings applied later on Al1230 plating.



Measuring organic coating thickness
According to the data obtained, the layer thicknesses described in Table 4 clearly shows a marked difference in thickness between the coatings applied to1 and 2 layers of resin (Hetron 197 -3 and Vipel F010).


The 1-layer covered around 200 (microns) while the 2 layer was about 300 (microns) thick. It can thus be seen that in fact the number of layers applied was the only factor having a significant effect on final coating thickness, regardless of the type of resin or pre-treatment.
The resins were applied in the same shape, using the same proceeding; their chemical composition had comparable specific densities and resin viscosities.
Tafel polarization curves
Figure 4 shows the Tafel polarization curves for the coatings obtained using the 3 types of resin and different types of anchor (mechanical and chemical). Table 4 shows the polarization curves obtained and anode and cathode slope values in each case, together with values for current density, corrosion potential and corrosion rate for each case studied.
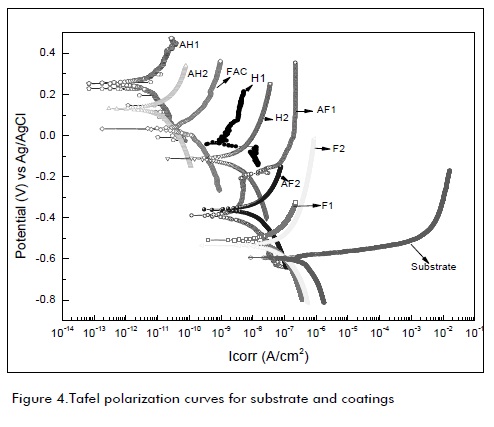
The polarization curve parameters were then used with the Stern -Geary (equation 1) equation for finding corrosion current density. It was determined that all coatings had good performance because they decreased current density value compared to that for the substrate. The coatings had a lower corrosion density value called AH with 1 and 2 layers, followed by those used in the military aviation industry. H2 and H1 coatings had a similar pattern to PIM. The coatings having a simple mechanical anchoring treatment with size 200SiCaasive paper had a significant increase in corrosion density compared to the PIM system; this was because the Alodine 5700 pre-treatment boosted the effect of surface passivation produced by plating and promoted a better adhesion pattern, both producing a rough surface by chemical bonding due to the presence of organic ligands in chemical oxidation. On the other hand, mechanical grinding with aasive paper No. 200only promoted rough surface; it could also have destroyed part of the thin sheet plating (AA1230)acting as a further barrier.
 | (1) |
The corrosion potential (Fig. 4) of each coating was the passive state of all coatings compared to the substrate, indicating good performance against corrosion in this type of resin. The substrate bias curve showed an increase in current density with small increases in potential creating a general solution on an accelerated basis. Other polarization curves indicated small increases in current density as the potential increased, indicating a low corrosion rate in the coatings being evaluated.
The formation of impedance spectra in Nyquist plot for both PIM specimen and AH, H, AF and F are plots made up of 2 semicircles. These systems are represented by an equivalent circuit described in Figure 5.

This equivalent circuit was applicable for coatings exposed to a 3.5%wtNaClsolution Rs represented electrolyte resistance in the case of the coatings of interest, Cc was coating layer capacitance and Rpo was resistance at the solution-coating interface in paint -based systems, referring to the coating formed by the Alodine 5700system, epoxy primer and paint finish, and in the case of synthetic resin AH1-based systems, it concerned the system consisting of Alodine 5700, antacid Hetron197 primer and resin-3.Increased Ccwas a direct relationship between the level of coating degradation while a decrease in Rpon was related to increased water absorption or permeability. The first semi-circle referred to organic coatings´ resistance (epoxy primer, polyurethane paint and synthetic resins) since conversion coating resistance was relatively small compared to that of organic coatings and therefore its contribution to impedance in the corrosion protection system was not clearly recognized. The coating having the highest polarization resistance value was AH2 (1x109 kΩ- cm2) and PIM lining (1x108 kΩ-cm2).EIS impedance spectra (Fig. 6) were obtained from Tafel polarization curves because coating surface treatment with Alodine 5700offered better anti-corrosion properties. The antacid 197-3 Hetron polyester resin had a more compact and dense molecular structure than Vipel F010 resin, resulting from its thermosetting nature. Ineeding and crosslinking between polymer chains by secondary bonds decreased polymer network free volume and thus reduced electrolyte ion species´ mobility through the coating to the substrate-coating interface

Conclusions
Plating substrate pre-treatment (AA1230) with a conversion coating using chemical oxidation created better corrosion resistance and adhesion to organic coating sthan mechanical surface treatment.
Clorendico Hetron 197-3 polyester resins used as final coating and barrier promoted a protection system having greater protection mechanisms due to their chemical barrier and compact, and dense molecular structure which decreased water and oxygen molecule permeability and ion specie mobility at the coatingsubstrate interface.
Hetron 5700 + 197 Alodine coating was superior to the normally-implemented system (PIM), representing added value in terms of relative low cost and ready availability on the market. Continued study of such configurations in synthetic resin coatingsis therefore of great interest.
References
Airframe & Powerplant Mechanics., Airframe Hanbook, U.S Departament of transportation., Federal Aviation Administration.,2008, pp. 45-62. [ Links ]
Atta, A,M., Elsaeed, A,M., Synthesis of unsaturated polyester resins based on rosin acrylic acid adduct for coating applications., Reactive & Functional Polymers, Vol. 67, No. 6, March 30., 2007, pp. 549-563. [ Links ]
Atta, A,M, Elsaeed, A,M, New vinyl ester resins based on rosin for coating applications., Reactive & Functional Polymers, Vol 66 No. 12, June 14., 2006, pp. 1596-1608. [ Links ]
Atta, A, M., Nassar, I.F., Unsaturated polyester resins based on rosin maleic anhydride adduct as corrosion protections of steel., Reactive & Functional Polymers, Vol, 67, No, 7, April, 2007, pp. 617-626 [ Links ]
Bierwagen, G.P., Tallman, D.E., Choice and measurement of crucial aircraft coatings system properties., Progress in Organic Coatings, Vol. 41 No. 4, Jan., 2001,pp. 201-216. [ Links ]
Buchheit, R.G., Guan, H., Active corrosion protection and corrosion sensing in chromate-free organic coatings., Fontana Corrosion Center, Progress in Organic Coatings, Vol. 47, No. 4, Oct., 2003, pp. 174-182. [ Links ]
Hanbook ASM., Metallography and Microstructures., ASM international, the materials information society, Vol. 09, 2009, pp.1-109. [ Links ]
Oñate, A. E, Las Aeronaves y sus Materiales. Tecnología Aeronáutica. Edición 1. Paraninfo (Ed), Madrid España., 1991, pp. 123-175. [ Links ]
Osborne, J,H., Testing and evaluation of nonchromated coating systems for aerospace applications., Progress in Organic Coatings, Vol. 41, No. 4, May 15, 2001, pp. 217-225. [ Links ]
Neuder, H., Sizemore, C., Molecular design of in situ phosphatizing coatings (ISPCs) for aerospace primers., Progress in organic coatings, vol. 47, No 3-4, March 4, 2003, pp 307 - 311. [ Links ]
Reporte de la ESTCP., Environmental Security Technology Certification Program., Non-Chromate Aluminum Pretratments, August 2003, Virginia, Oct ., 2004, pp. 1-110. [ Links ]
Reynolds, L.B., Twite, R., Preliminary evaluation of the anticorrosive properties of aircraft coating by electrochemical methods., Progress in Organic Coatings, Vol. 32 No.1-4, April 7, 1997, pp. 31-34. [ Links ]
Sanchez-Amaya, J.M., Osuna, R.M., Monitoring the degradation of a high solids epoxy coating by means of EIS and EN, Progress in Organic Coatings, Vol. 60 No 3, July 31, 2007, pp. 248-254 [ Links ]
Sathiyanarayanan, S., Azim, S., Corrosion protection coating containing polyaniline glass flake composite for steel., Electrochemical Acta, Vol. 53 No5, January 1, 2008, pp. 2087- 2094. [ Links ]
Twite, R.L., Bierwagen, G.P., Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys., Progress in organic coatings, Vol. 33, No 2, Feb 23, 1998, pp. 91-100. [ Links ]
Z.H. Fang, H.Y. Duan, Z.H. Zhang, J. Wang, D.Q. Li, Y.X. Huang, J.J. Shang, Liu., Z.Y., Novel heat-resistance UV curable waterborne polyurethane coatings modified by melamine., Applied Surface Science Vol. 257, No. 11, 15 March 2011, pp. 4765-4768. [ Links ]











 text in
text in 


