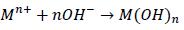Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.32 no.1 Bogotá Jan./Apr. 2012
Corrosion resistance of Cu-Al coatings produced by thermal spray
Resistencia a la corrosión en recubrimientos Cu-Al producidos con el sistema de proyección térmica por llama
Laura Marcela Dimaté Castellanos1, Jhon Jairo Olaya Flórez2, and José Edgar Alfonso Orjuela3
1 Physics Engineering and MSc in Materials and Processes, Universidad Nacional de Colombia. lmdimatec@unal.edu.co.
2 MSc in Materials and Processes, Universidad Nacional de Colombia, Colombia. PhD in Engineering, Universidad Nacional Autónoma de México, Mexico. Associated Professor, Universidad Nacional de Colombia. jjolayaf@unal.edu.co.
3 MSc in Science - Physics, Universidad Nacional de Colombia. PhD in Sciences-Physics, Universidad Autónoma de Madrid, España. Associated Professor, Univer-sidad Nacional de Colombia. jealfonsoo@unal.edu.co.
Received: August 1st 2011; Accepted: February 27th 2012
RESUMEN
Muchas de las piezas en la industria naval están fabricadas con aleaciones a base de cobre. Con el paso del tiempo estas piezas se degradan debido a los procesos de corrosión que se generan por el ambiente marino. Estas piezas se pueden recuperar mediante ingeniería de superficies, haciendo uso de la deposición de recubrimientos adecuados. En este trabajo se estudia la influencia de tres métodos en la preparación de la superficie de sustratos de bronce fosforado sobre la resistencia a la corrosión de recubrimientos comerciales de marca Proxon 21071 con composición química Cu+11% Al-Fe. La preparación de la superficie se realizó mediante tres métodos: granallado con arena, granallado metálico con alúmina y pulimento con disco abrasivo, con y sin capa base. Los recubrimientos depositados se caracterizaron microestructuralmente por medio de difracción de rayos X (XRD), microscopía óptica y microscopía electrónica de barrido (SEM). La resistencia a la corrosión se evaluó por medio de pruebas electroquímicas a través de la prueba de espectroscopía de impedancia electroquímica (EIS). En general, las superficies prepara-das con granallado presentaron la mejor resistencia a la corrosión, de tal suerte que estos sistemas pueden ser una alternativa viable para recuperar cierto tipo de piezas en la industria naval. Adicionalmente fueron discutidos los mecanismos de corrosión de los recubrimientos producidos.
Palabras clave: Cu-Al, proyección térmica por llama, resistencia a la corrosión, impedancia.
ABSTRACT
Many components in the shipbuilding industry are made of copper-based alloys. These pieces tend to break due to corrosion generated by a marine environment; such components can be salvaged through surface engineering, through deposition of suit-able coatings. This paper studied the influence of three surface preparation methods involving phosphor bronze substrates concerning the corrosion resistance of commercial coatings having Al-Cu +11% Fe chemical composition. The surface was prepared using three methods: sand blasting, shot blasting and metal polishing with an abrasive disk (with and without a base layer). The deposited coatings were micro-structurally characterised by x-ray diffraction (XRD), optical microscopy and scanning electron microscopy (SEM). Corrosion resistance was evaluated by electrochemical test electrochemical impedance spectroscopy (EIS). Surfaces prepared by sandblasting showed the best resistance to corrosion, so these systems could be a viable alternative for salvaging certain parts in the marine industry. The corrosion mechanisms for the coatings produced are discussed in this research.
Keywords: Cu-Al, thermal spray flame, corrosion resistance.
Introduction
Thermal spraying in the area of surface technology has emerged in recent years as a low cost technique having excellent component recovery results (Thorpe 1993). Many constituent components deteriorate in the shipbuilding industry's harsh environmental conditions in terms of wear and resistance to corrosion. Some of these pieces are not mass produced, leading to high acquisition costs and long waiting periods, causing significant delays because of higher maintenance downtime.
Copper alloys are mainly used for oil and gas pipelines, heat ex-changers, propellers, valves, gate valves and laminated plates for small boats' steel hulls. Copper alloys are characterised by having good resistance to corrosion in a marine environment. However, they corrode in certain conditions such as high flow of sea water, when exposed to certain contaminants in water and also when the requirements for a suitable chemical composition in the alloy are non-standard. Copper alloys are not usually protected by paint coatings (Féron 2007).
Aluminium bronzes consist of a family of copper-based alloys containing 5% to 12% aluminium and have excellent resistance to corrosion in a wide range of conditions. They also have excellent adhesion which, combined with good resistance to oxidation, low cost and high deposition rate, makes these alloys a candidate for coating applications to enhance and restore the parts exposed to saline environments (Zhang et al., 2006).
Thermal spraying has been developed as a technology for produc-ing good quality coatings on various surfaces. These are based on the deposition of molten or semi-molten material at high speed on a substrate to produce a coating (Bradai et al., 2008). This tech-nique's main objective is to provide the elements with increased life, with an extensive list of materials to be deposited and coating substrates. The speed and temperature at which the particles collide with the substrate define adhesion, porosity, hardness, surface roughness, wear resistance and resistance to corrosion (Uyulgan et al., 2007).
This paper evaluates the deposition of a self-fluxing alloy of alu-minium-bronze for use in marine applications using thermal spray-ing flame. This coating has HRB 60hardness, its chemical composi-tion is Cu + 11% (Al, Fe), it has -140 + 325 mesh particle size, around 3,000 psi adhesion and maximum 370°C service tempera-ture (Technical Data sheet. Proxon 21071, 2000). To this end, aluminium bronze coatings were deposited on brass substrates following different surface preparations to establish their behaviour regarding corrosion in a saline medium simulating the environment to which they would be subjected to during an operation to re-cover components, aimed at implementing this system (coating-thermal spray) in a company laboratory.
Experimental development
Surface preparation
The substrates used were phosphor bronze in 12.5 mm diameter, 3 mm thick cylinders polished with 80 um grain size sand on substrate surface preparation to clean the surface and achieve appropriate roughness for improved coating adhesion. The follow-ing preparation methods were used. Shot blasting involved Al2O3 in two stages. Alumina was used in the first phase with 100 um particle size, at 100 psi pressure for 10s, then 500 um particles in the same pressure and time conditions.
The surface was also prepared by sand blasting using commercial sand with 200 um particle size, 100 psi pressure and 10s. The surface was prepared with an abrasive disc grinder for 10 s. A degreaser was applied after surface preparation and dried with pressurised air.
Coating deposition
21021 Proxon coatings were produced by thermal spray with flame (using powders) with a CastoDyn 8000 gun (i.e. modular acetylene equipment). The coatings were deposited on phosphor bronze and on Ni-Al base layer deposited on phosphor bronze used in industry to improve system adhesion. Similar deposition conditions were used for base layer deposition and the coating. 4 bars oxygen pressure, 0.7 bars acetylene pressure, 0.6 bars com-pressed air pressure and 150 mm projection distance were used. Substrate temperature was around 120°C. The coatings were deposited with about 80 um thickness and 10 to 15 um base layer.
Microstructural characterisation
The coatings were structurally characterised by x-ray diffraction using PANalytical X-pert Pro equipment working at a grazing angle with copper Ka (1.540998 Å) operating at 45 kV and 40 mA. Thickness and porosive quality were measured by Leco light mi-croscope with convex lens, through coatings' cross-section. The coatings' surface was studied by scanning electron microscopy with FEI QUANTA 200 in a high vacuum and working at 30kV voltage. Morphological analysis of the coating surface to find Ra and Rsa roughness values were obtained by confocal laser micro-scope using a Zeiss LSM 700 system.
Evaluating resistance to corrosion
Electrochemical impedance spectroscopy tests evaluated corrosion resistance using a Gamry Reference 600 potentiostac-galvanostat following ASTM G5 recommendations. A 3% NaCl solution was used at room temperature in the electrochemical test; initial fre-quency was 100 MHz and 0.01 kHz at the end, with 10 (mV) AC voltage perturbation. The area of the sample exposed to the solu-tion was 0.196 cm2. The measurements were made after 1, 24, 48, 72 and 168 hours of the sample being immersed in the solu-tion to evaluate the different coatings' corrosion behaviour. The EIS test results were modelled on an equivalent electrical circuit with Gamry-Echem analysis software.
Results and Discussion
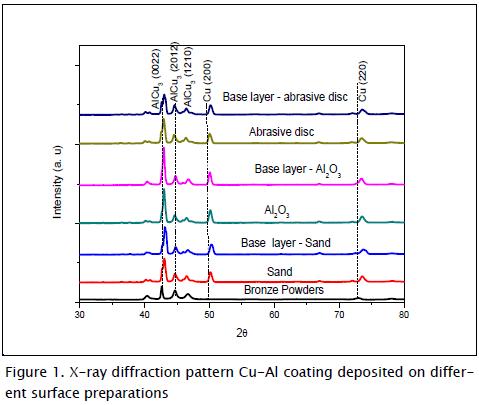
Figure 1 shows the brass coating's x-ray diffraction pattern on the substrate's three prepared surfaces, with and without base layer. The cubic structure of copper was observed in all cases and the AlCu3 compound's clear orthorhombic structure pattern at 27.16, 40.28, 42.73, 44.88, 78.30 and 76.89 peaks. These results agreed with those found by other researchers (Liang et al., 2000).
Figure 2 shows the micrograph of aluminum bronze coating de-posited on phosphor bronze substrates prepared with alumina and base layer. Un-melted particles were observed, having porosity and micro-cracks which were due to rapid cooling in coatings with powdered material used in thermal flame spray system (Uyulgan et al., 2007).
Dendritic or star shaped structures were also observed which were due to low substrate temperatures during deposition (Dhiman et al., 2007).
Figure 3 shows a micrograph of an aluminium bronze coating cross-section in secondary electron mode at 500X. The layered structure typical of these coatings and the interface substrate / coating dark areas can be observed, which could be interpreted as gaps reducing adhesion (Uyulgan et al., 2007), surely reducing the entire system's mechanical properties (Kumar et al., 2006). Oxides may not have formed at the interface because the aluminium-bronze coating binding mechanism is related to aluminium's affin-ity for oxygen which promotes particle surface exothermic oxida-tion and removes substrate surface oxides (Dorfman 2005).
Figure 4 shows the EIS tests for all preparations used at 168 h. A time constant for the phase diagram was clearly observed and a small curve at low frequencies. Higher impedance values were shown in the coatings deposited on the surface prepared with alumina, followed by sand, and the lowest appeared in the surface prepared with polishing or abrasive disc. This behaviour could have been related to compressive residual stresses induced by shot blasting on the surface, being possible because of the change in physicochemical properties and thus surface reactivity and/or conductivity (C. Aparicio 1999).
The coatings deposited on the base layer had higher impedance values. This behaviour appeared to be due to the presence of the Ti-Al-based layer, an interface that can act as a further barrier inside the coating, thereby preventing interconnected spread. Furthermore, the base coating layer was more compact and had less oxide content and better intra-layer cohesion compared to the brass coating (Lekatou et al., 2008, Xu et al.).
Figure 5 shows a Bode diagram of the coating on a surface pre-pared with alumina and a base layer, characterised by having the best performance against corrosion. It was observed in the earlier trials that impedance values decreased with contact with NaCl electrolyte, possibly due to electrolyte diffusion to the substrate through the grain boundaries between the splats, corrosion being controlled by mass transfer (Tao et al., 2010). The influence of the defects and un-melted particles or grain boundaries presented in the coatings deposited by thermal spraying on the behaviour of resistance to corrosion thus became evident, enabling the electrolyte to easily penetrate the coating to reach the substrate (Lister et al., 2002).
However, impedance increased again after about 24 hours testing; this involved reactant or corrosion product diffusion through the layer for defects to form, such as the pores characteristic of these coatings (Xu et al., 2011).
Figure 6(a) shows a SEM micrograph of the brass coating prepared with alumina grit and base layer. Some corrosion products ad-hered to the coating. Corrosion products adhered well to the oxides formed during thermal spray coating deposition with flame (MH Pombo Rodriguez et al., 2007).
This behaviour has also been reported by Galvele et al., (Galvele, 1976) who proposed that passive layer breakdown and corrosion can begin in the oxide layer formed during deposition. Corrosion can also become accelerated due to the fact that electrolyte solu-tion diffusion can attack the substrate below the oxide layer. Figure 6(b) shows a micrograph of the SEM cross-section of the brass coating and base layer prepared with alumina grit after being subjected to test impedance for 168 h. The micrograph shows that the electrolyte has penetrated to the substrate surface to become delaminated. A coating layer was observed on the surface; it was probably formed by corrosion products (Lekatou et al., 2010).
The experimental impedance results could be modelled along with the circuit shown in Figure 7. This circuit has also been proposed by Dermaj et al., (Dermaj et al., 2007) and Rahmouni et al., (Rah-mouni et al., 2005) for bulk bronze subjected to corrosion in an NaCl solution. There were two parallel circuits in this circuit. CPE1 described the coating surface's dielectric nature in the first circuit (CPE1, RPO) and RPO represented resistance to charge transfer through coating porosity. The other parallel circuit (CPE2, RTC) described substrate behaviour (at the interface) in contact with the electrolyte, where CPE2 was the double layer constant phase element between the substrate and the solution, and RTC was resistance to charge transfer at the substrate / coating interface. Rsln was resistance to the solution (Dermaj et al., 2007).
Table 1 contains the measured roughness arithmetic values (Ra). It was observed that the lower roughness coatings were blasted with sand, then prepared by polishing and the surface prepared with Al203 blasting. It was also observed that adding the base layer caused a slight increase in final coating roughness. Figure 8 shows the image taken with the confocal microscope for the bronze coating deposited on a surface prepared with blasting alumina and base layer, having undergone the impedance test for 168 h. Over-all, a slight increase in roughness was observed after the corrosion test; however, noticeable degradation was not observed on surface corrosion products.
Given the above results, a mechanism was proposed for under-standing the corrosive phenomena shown by the whole coating / substrate system. Initially, it had to be born in mind that the coat-ings deposited with flame sprayed material had inherent porosity and defects, such as un-melted particles and / or semi-melted, oxides and micro-cracks allowing the electrolyte to diffuse to the surface or substrate. Diffusion was initiated by the passage of electrolyte Cl ions through the transition zone, extending from coating surface to the interface. A cathodic reaction was thus created which occurred at the coating surface and an anodic reaction at the substrate surface, involving the following reactions:
Corrosion was complemented by the ions produced by the anodic reaction moving through the pores of the coating and combining with the OH-products produced in the cathode reaction, as de-scribed in the following reaction:
However, a reaction could occur on the substrate surface with the positive ions present in the anode area and Cl ions to maintain electrical neutrality. Large amounts of MCln (M refers to the metal atoms in the reaction) were thus formed at the interface, through the following reaction:
H+ ions such as Cl- diffusing to the substrate through pores or the borders of the splats may accelerate substrate corrosion, whereas corrosion products accumulate in the coating defects. Increased tensile forces thus leads to coating delaminate, generating prefer-ential substrate corrosion under the coating (Zhao et al., 2005).
Corrosion resistance can be influenced by the combination of two important properties: roughness and adhesion. The surfaces of coatings exhibiting greater roughness have more preferential sites for developing selective corrosion or pitting. This is because the rougher surface has peaks and valleys which act as active sites and promote corrosion through their sharp edges (Çelik et al., 1999). However, coating adhesion largely depends on roughness. In the substrate on surfaces having low roughness, the splat does not allow binding to occur between the particles and the projected substrate due to rapid cooling and solidification. The mechanical connection cannot therefore be effectively created regarding the action of thermal contraction during projected droplet solidifica-tion (Wang et al., 2005).
The surface prepared with Al203 blasting in this paper had the best corrosion resistance. This result may indicate that adhesion was more important than roughness for determining these coatings' corrosion resistance. Figure 9 shows electrolyte propagation in the coating prepared with alumina blasting. Electrolyte penetration to the substrate can be seen and a few areas at the interface having coating detachment (black areas), whereas better adherence was attributed to the higher roughness value.
Corrosion rate was reduced in the coatings deposited on the base layer because the electrolyte penetration path was greater since it had to diffuse through the pores, the boundaries of the splats and other coating defects and the base layer. The corrosive solution thus propagated through the coating / base and base / substrate interface. It should also be kept in mind that the base layer coating behaved more nobly than the bronze substrate and, once the electrolyte reached the substrate surface, it created a galvanic coupling between both systems; it thus produced preferential corrosion in the substrate, as illustrated in Figure 10.
Conclusions
Coatings were deposited by using thermal spraying of powdered material to the flame by varying the surface preparation for differ-ent quality coatings, thereby demonstrating the importance of surface preparation for resistance to corrosion.
The coatings had a Cu-Ni laminar microstructure with imperfec-tions such as pores and micro-cracks, un-melted particles and semi-melted particles. The formation of splats was evident. The surface preparation method had no appreciable effect on the coatings' final microstructure.
It was established that alumina blasting was the best surface prepa-ration method as it provided greater coating roughness, increasing its adherence and thus making it harder for the coating to delami-nate after the electrolyte had penetrated the coating in the corro-sion tests.
The coating corrosion mechanism involved the pores and defects in the material, allowing the electrolyte to penetrate down to the substrate, and was dependent on the roughness, solution dis-placement through the substrate and adhesion to the interface. The coatings' corrosion resistance was strongly influenced by the coatings' porous microstructure. However, considering that these coatings application is primarily for dimensional recovery, these are thus viable alternatives for application in the shipbuilding industry, in conditions regarding appropriate surface preparation.
Acknowledgments
The authors would like to acknowledge the financing provided by Colciencias (Project No. 778) and Cotecmar for this project.
References
Bradai, M. A., Bounar, N., Benabbas, A., Ati, A., Study of micro-structure, phases and microhardness of metallic coatings deposited by flame thermal spray, Journal of Materials Processing Technology, Vol. 200(1-3), 2008, pp.410-415. [ Links ]
C. Aparicio, D. R., F.J Gil, C. Fonseca, M. Barbosa, G. Nuss-baum, A. García, J.A Planell, Comportamiento a la corrosión de implantes de titanio granallados. Biomecánica, VoI 13, 1999, pp. 21-26. [ Links ]
Çelik, E., DemirkIran, A. S., Effect of grit blasting of substrate on the corrosion behaviour of plasma-sprayed Al2O3 coatings, Sur-face and Coatings Technology, Vol.116, No. 119, 1999 pp.1061-1064. [ Links ]
Dermaj, A., Hajjaji, N., Joiret, S., Rahmouni, K., Srhiri, A., Takenouti, H., Vivier, V, Electrochemical and spectroscopic evidences of corrosion inhibition of bronze by a triazole derivative, Electrochimica Acta, Vol 52, No. 14, 2007, pp. 4654-4662. [ Links ]
Dhiman, R., McDonald, A. G., Chandra, S. Predicting splat mor-phology in a thermal spray process, Surface and Coatings Technology, Vol. 201 No.18, 2007, pp 7789-7801. [ Links ]
Dorfman, M. R., Thermal spray coatings in K. Myer, (ed.), Hand-book of Environmental Degradation of Materials. Norwich, NY: William Andrew Publishing, pp. 405-422. [ Links ]
Eutectic & Castolin. (2000). "Technical Data sheet. Proxon 21071." [ Links ]
Féron, D.,Corrosion behaviour and protection of copper and aluminium alloys in seawater." Woodhead Publishing in Mate-rials(ed), 2007, pp. 5-8. [ Links ]
Galvele, J. R.,Trasport Processes and the Mechanism of Pitting Metals." Electrochemical Society, Vol. 123, 1976. [ Links ]
Kumar, S., Selvarajan, V., Padmanabhan, P. V. A., Sreekumar, K. P, Characterization and comparison between ball milled and plasma processed iron-aluminium thermal spray coatings, Surface and Coatings Technology, Vol. 201, 2006, pp. 1267-1275. [ Links ]
Lekatou, A., Zois, D., Grimanelis, D., Corrosion properties of HVOF cermet coatings with bond coats in an aqueous chloride environment, Thin Solid Films, Vol. 516 No. 16, 2008, pp. 5700-5705. [ Links ]
Lekatou, A., Zois, D., Karantzalis, A. E., Grimanelis, D, Electro-chemical behaviour of cermet coatings with a bond coat on Al7075: Pseudopassivity, localized corrosion and galvanic effect considerations in a saline environment, Corrosion Science, Vol. 52 No. 8, 2010, pp. 2616-2635. [ Links ]
Liang, W., Xiaolei, X., Jiujun, X., Zukun, H., Microstructures and properties of PVD aluminum bronze coatings, Thin Solid Films, Vol 376, No. 1-2, 2000, pp 159-163. [ Links ]
Lister, T., Wright, R., Pinhero, P., Swank, W, Corrosion of thermal spray hastelloy C-22 coatings in dilute HCl." Journal of Thermal Spray Technology, Vol. 11 No. 4, 2002, pp 530-535. [ Links ]
M. H. Pombo Rodriguez, R., Paredes, R. S. C., Wido, S. H., Calixto, A., Comparison of aluminum coatings deposited by flame spray and by electric arc spray, Surface and Coatings Technology, Vol. 202 No. 1, 2007, pp 172-179. [ Links ]
Marulanda, J, Rociado Térmico, Colombia, sin publicar, pp. 45-47 [ Links ]
Rahmouni, K., Keddam, M., Srhiri, A., Takenouti, H., Corrosion of copper in 3% NaCl solution polluted by sulphide ions, Corrosion Science, Vol. 47 No. 12, 2005, pp. 3249-3266. [ Links ]
Tao, Y., Xiong, T., Sun, C., Kong, L., Cui, X., Li, T., Song, G.-L., Microstructure and corrosion performance of a cold sprayed aluminium coating on AZ91D magnesium alloy, Corrosion Science, Vol. 52 No. 10, 2010, pp. 3191-3197. [ Links ]
Thorpe, M. L. ,Thermal Spray: Industry in Transition, Adv.Mater. Process., Vol 143 No. 5,1993, pp. 50-56. [ Links ]
Uyulgan, B., Dokumaci, E., Celik, E., Kayatekin, I., Ak Azem, N. F., Ozdemir, I., Toparli, M., Wear behaviour of thermal flame sprayed FeCr coatings on plain carbon steel substrate, Journal of Materials Processing Technology, Vol. 190 No. 1-3, 2007, pp- 204-210. [ Links ]
Wang, Y. Y., Li, C. J., Ohmori, A., Influence of substrate roughness on the bonding mechanisms of high velocity oxy-fuel sprayed coatings, Thin Solid Films, Vo. 485 No.1-2, 2005, pp.141-147. [ Links ]
Xu, C., Du, L., Yang, B., Zhang, W., Study on salt spray corrosion of Ni-graphite abradable coating with 80Ni20Al and 96NiCr-4Al as bonding layers, Surface and Coatings Technology, In Press, Corrected Proof. [ Links ]
Zhang, Z.-l., Li, D.-y., Wang, S., High temperature performance of arc-sprayed aluminum bronze coatings for steel, Transactions of Nonferrous Metals Society of China, Vol. 16 No. 4, 2006, pp 868-872. [ Links ]
Zhao, W.-M., Wang, Y., Dong, L.-X., Wu, K.-Y., Xue, J., Corrosion mechanism of NiCrBSi coatings deposited by HVOF, Surface and Coatings Technology, Vol. 190, 2005, pp 293-298. [ Links ]