Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.33 no.3 Bogotá Sept./Dec. 2013
D. Moncayo1, G. Buitrago2 and N. Algecira3
1Diana Cristina Moncayo Martinez. BSc Chemical Engineering. Maestría en Ciencia y Tecnología de alimentos. Affiliation: Universidad Nacional de Colombia, Colombia. E-mail: dcmoncayoma@unal.edu.co
2Gustavo Buitrago Hurtado. BSc Chemical Engineering. Affiliation: Biotechnology Institute. Universidad Nacional de Colombia, Colombia. E-mail: gbuitragoh@unal.edu.co
3Néstor Ariel Algecira Enciso. BSc Chemical Engineering. Universidad Nacional de Colombia, Colombia. Affiliation: Facultad de Ingenieria Química y Ambiental, Universidad Nacional de Colombia, Colombia. E-mail: nalgecirae@unal.edu.co
How to cite: Moncayo, D., Buitrago, G., Algecira, N., The surface properties of biopolymer-coated fruit: a review., Ingeniería e Investigación, Vol. 33, No. 3, December 2013, pp. 11 - 16.
ABSTRACT
Environmental conservation concerns have led to research and development regarding biodegradable materials from biopolymers, leading to new formulations for edible films and coatings for preserving the quality of fresh fruit and vegetables. Determining fruit skin surface properties for a given coating solution has led to predicting coating efficiency. Wetting was studied by considering spreading, adhesion and cohesion and measuring the contact angle, thus optimising the coating formulation in terms of biopolymer, plasticiser, surfactant, antimicrobial and antioxidant concentration. This work reviews the equations for determining fruit surface properties by using polar and dispersive interaction calculations and by determining the contact angle.
Keywords: Edible coating, edible film, contact angle, wettability.
RESUMEN
La elaboración de películas y recubrimientos con materiales naturales y biodegradables, disminuyen los daños ambientales comparados con los ocasionados por materiales comunes de plásticos sintéticos. Las formulaciones con biopolímeros como celulosa, gomas, almidones o proteínas; permiten conservar la calidad de las frutas y los vegetales frescos. La determinación de las propiedades que tiene la superficie de la piel de la fruta y la solución de recubrimiento, permiten predecir la eficiencia del proceso. El estudio del fenómeno de humectabilidad, considerando el trabajo de expansión, cohesión, adhesión y medición del ángulo de contacto, logra optimizar la formulación de recubrimiento en cuanto a concentraciones de biopolímeros, plastificantes, surfactantes, antioxidantes, etc. Este trabajo es una revisión de las ecuaciones que permiten determinar las propiedades superficiales de las frutas, considerando el cálculo de las interacciones polares, dispersivas y la determinación del ángulo de contacto.
Palabras clave: recubrimiento comestible, película comestible, ángulo de contacto y humectabilidad.
Received: December 5th 2012 Accepted: October 18th 2013
Introduction
Fruit and vegetables are live tissues having high moisture content (60%-95%) and which lose water and continue respiration thereby producing heat and water at the expense of food reserves (Mishra and Gamage, 2007). Fresh products cannot continue replenishing carbohydrates or water after harvesting. Plants use stored starch or sugar in respiration and will stop when such reserves become exhausted. Consequently, ageing begins, culminating in product death and decay (FAO, 1993).
Decay is primarily caused by weight-loss, not only through direct quantitative loss but also through the deterioration of appear-ance, textural quality (softness, loss of turgidity and juiciness), and nutritional quality. Transpiration rate (evaporation of water from plant tissues) is influenced by internal or intrinsic factors (morphological and anatomical characteristics, surface lesions, and maturity stage) (Barreiro and Sandoval, 2006) and environmental factors (temperature, relative humidity, air movement and atmospheric pressure) (Kader, 2002; Ulloa, 2007).
Packaging is widely used for preserving, distributing and market-ing fruit and vegetables and is often used in combination with other preservation methods (Hoover, 1997). However, the disposal of packaging materials leads to ecological problems and additional recycling costs (Tzoumaki et al., 2009; Viña et al., 2007). Edible coatings are one of the most innovative strategies for extending fruit and vegetable shelf-life life; such coatings act as barriers to gas transport and produce similar effects to stor-age in a controlled atmosphere (Park, 1999).
Edible coatings and edible films are terms which are frequently interchangeably regarding food packaging; however, a distinction must be made between the two terms. A film is a thin skin which has been formed, for example, by casting a biopolymer solution separately from the food; this film can be later applied to the food. By contrast, a coating is a suspension or an emulsion which is applied directly to the food surface, and later becomes transformed into a film (Souza et al., 2010).
Edible coatings are usually made from materials such as proteins, lipids and polysaccharides; the main polysaccharides used in this are starches and modified starches, cellulose derivatives, chitosan, pectin, alginate and other gums (Hernandez-Izquierdo and Krochta, 2008; Tzoumaki et al., 2009). Thin edible films act as barriers to external elements (such as moisture, lipids and gasses) and improve mechanical properties during handling, transportation and may also serve as food additive carrier. Films also prevent the loss of and even increase volatile flavour production, thus extending product postharvest shelf-life (Azeredo et al., 2012; Durango et al., 2011; Guilbert et al., 1996; Oliva and Barbosa-Cánovas, 2005; Quintero et al., 2010; Ramos et al., 2013).
Most edible films and coatings contain at least one high molecular weight polymer. Large chain polymeric structures are required for creating matrices having suitable cohesive strength. The films so obtained usually have reduced flexibility and are porous and permeable to gasses, vapour and solutes. A uniform distribution of polar groups throughout the polymer chain increases coating material ability to form hydrogen bonds and participate in ionic interactions (Kester and Fennema, 1986).
Typical coating-forming methods would include spray-coating, and dipping (Dangaran et al., 2009). The choice of method de-pends on coating solution concentration and a coating's ability to form a thin layer which develops into a protective film on fruit surface, upon drying (Pavlath and Orts, 2009).
Edible coatings represent a novel approach to preserving the quality of characteristics such as fresh or minimally-processed products' colour, texture, antioxidant properties and freshness, thus extending product shelf-life (Ali et al., 2010; Chiumarelli and Hubinger, 2012; Das et al., 2013; Fan et al., 2009; Gounga et al., 2007; Qi et al, 2011; Robles-Sánchez et al, 2013). It has been reported that chitosan (a chitin derivative) can form excellent films having antimicrobial properties; it has been widely used in controlling weight-loss in fresh strawberries (Fragaria x ananassa) and raspberries (Rubus idaeus) (Han et al., 2004; Park, 1999; Ribeiro et al., 2007; Vargas et al., 2006), mango (Mangifera indica), litchi (Martínez-Castellanos, 2009), blueberries (Duan et al., 2011) and other fruit and vegetables (Lin and Zhao, 2007). Coat-ings consisting of caseinates and milk proteins provide an excel-lent barrier to oxygen and have been studied for controlling postharvest respiration in apples (Malus sylvestris) (Letien et al., 2001) and strawberries (Vachon et al., 2003).
The effectiveness of fruit and vegetables' edible coatings primarily depends on controlling coating solution wettability which affects a film's coating thickness (Park, 1999). The coating should be able to wet and spread uniformly over product surface and, upon drying, a coating should have suitable adhesion, cohesion and durability to function properly. Coating involves the wetting of the product to be coated by the coating solution, which may penetrate into the fruit skin (Hershko et al., 1996, Krochta and Mulder-Johnston, 1997), followed by possible adhesion between these two commodities.
The aim of this review is to describe the concepts for characterising the surface properties of fruit coated by biopolymers and to describe the effects of plasticiser type and concentration, as well as surfactant and polymer concentration, on these surfaces' coating wettability.
Surface properties
Optimum wettability requires the greatest possible area for contact between a solution and the surface to be coated, thus avoiding the disruption of air between the solution and the surface. The film is expected to be heavy and continuous on a non-porous surface; therefore, the film must have good cohesion. The adhesion between the final dried film and the food is extremely important; a layer of the dried, solid film should attach to the food, particularly in surface regions where discontinuities may exist. Wettability can be characterised in terms of two physical parameters: interfacial tension and contact angle (Marzo-Rojas, 2010).
Wettability
Wettability determines the spreading coefficient (Ws), the work of adhesion (Wa) and the work of cohesion (Wc). Adhesion strength promotes liquid expansion on a solid surface, and cohesive hardness promotes liquid contraction on a solid surface. Wettability behaviour is determined by the equilibrium between these forces. The surface tension of a coating solution is typically determined using a pendant drop technique, together with the Young-Laplace equation (Song and Springer, 1996).
Contact angle (θ)
Wettability property determination is essential in characterising the surface of packaging materials. The wettability of a solid surface can be determined in a relatively simple manner by contact angle measurement (Kiely and Olson, 2000). When a liquid drop is placed on a smooth, flat solid surface, the liquid forms a thin film or droplet (sessile drop) on the surface. The droplet makes a finite contact angle with the surface (Figure 1). The magnitude of the contact angle depends on the attractive force between the solid and the liquid and on the surface tension of the liquid (Tracton, 2005).

The contact angle (θ) of a liquid droplet on a solid surface is determined by the mechanical equilibrium of the droplet regarding the action of three interfacial tensions: solid-vapour (γSV), solid-liquid (γSL) and liquid-vapour (γLV). The equilibrium spreading coefficient may be defined by equation (1-1) and can only have negative or zero values (Rulon and Robert,1995):

where Wa and Wc are the work of adhesion and cohesion, as defined by equations (1-2) and (1-3), respectively:

For θ < 90 o (or equivalently, for γSV > γSL), the liquid is said to wet the solid or the system is said to be wetting. For θ > 90o, the system is non-wetting; in this case, the liquid does not wet the solid. An extreme case occurs when θ approaches zero: the liquid expands on the solid and shows perfect wettability (Palacios, 1999; Skurtys et al., 2010).
Contact angle value is affected by the following factors:
- Droplet size. Some studies have stated that the contact angle should be measured on two sides of a drop (Drelich, 1996; Greiveldinger and Shanahan, 1997). As the surface of a specimen is heterogeneous, the arithmetic average of at least nine different drop measurements should be used as the test result. Furthermore, readings must be taken quickly to avoid volume becoming reduced by evaporation. Kaelble and Cirlin (1971) recommended that:
- Drop volume should be 28 to 0.5 mm3;
- Temperature should be up to 8oC. Temperature changes have a small effect on surface free energy. Normal temperature fluctuations which may occur during tests do not have a strong effect on contact angle value (Zouvelou et al., 2007);
- Regarding surface impurity, the appearance of hysteresis is usually attributed to roughness, its chemical heterogeneity, and/or surface active impurities present in the liquid (Chibowski et al., 2002); and
- Concerning surface roughness (thermodynamic hysteresis), it is generally accepted that if Ra< 0.5 µm, the effect that roughness has on the contact angle is insignificant (Rudawska, 2009).
Surface free energy
Surface free energy is a thermodynamic quantity associated with the equilibrium state of the atoms in a surface layer of matter. This quantity is characteristic for each substance and represents the specific state of non-equilibrium intermolecular interactions in the boundary phase between two media. There are many methods for determining a liquid's surface free energy and various indirect methods are used for determining the surface free energy of solids. Examples of indirect methods would include Fowkes, Owens-Wendt and Wu's methods. Other methods include the Zisman method, the Newman method and van Oss-Chaudhury-Good methods: the latter has become increasingly popular (Rudawska and Jacniacka, 2009). The Owens-Wendt method consists of determining dispersion and polar surface free energy components on the basis of the Bethelot hypothesis (Rudawska and Jacniacka, 2009). The Fowkes' method divides surface energy into dispersive and polar components and uses a geometric mean approach for combining their contributions (Lu et al., 2012). The Neumann method is based on the assumption that there is a relationship between surface energy free of a solid, surface energy free of the liquid wetting the solid surface and surface energy free of the solid-liquid interface (enkiewicz, 2007).
Surface tension
The surface tension of a liquid is defined as the amount of energy needed to increase the surface per area unit (J • m-2,). Surface tension may be equivalently defined as the normal force acting per unit length (N • m-1) of the surface. Surface tension results from the action of intermolecular forces on the interface (i.e., the separation plane between the two phases) and depends on the nature of the liquid, the surrounding medium and temperature. The surface tension of a particle has a non-polar component (i.e. the Lifshitz-van der Waals (LW) force), a polar component (the Lewis acid-base (AB) force) and an electrostatic component (Han et al., 2005). Surface tension is also used as a measure of adhesion properties and is determined by measuring the contact angle of a standard liquid on the surface. This method requires calculating the critical surface tension of the solids being studied (Casariego et al., 2008).
Cerqueira et al., (2009) derived a set of equations for calculating the surface energy of fruit in contact with a pure liquid, for known polar  and dispersive
and dispersive  interactions. If θ is the contact angle between the liquid and solid, the interactions can be expressed in terms of the reversible work of adhesion, Wa, as:
interactions. If θ is the contact angle between the liquid and solid, the interactions can be expressed in terms of the reversible work of adhesion, Wa, as:
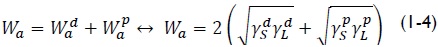
Rearranging (1-1) yields:

where  is an independent variable and
is an independent variable and 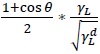 is the dependent variable in (1-5).
is the dependent variable in (1-5).
Table 1 shows the dispersion component of surface tension for pure liquids, which can be used for calculating the critical surface tension for fruit characterisation.

Critical surface tension (γc) is estimated by extrapolating a Zisman plot, obtained by plotting the cosine of the contact angle of pure liquids on a solid surface to be studied against the surface tension of the same series of liquid. The intercept of the curve at cos θ = 1 is known as critical surface tension. Critical surface tension is a fictitious value of γsv which is often used to describe surface wettability (Lima et al., 2010). Critical surface tension is thus defined as:

Choi et al., (2002) found that the surface tension of a chitosan solution decreased as the concentration of Tween 80 was increased. By contrast, interfacial tension (γSL) between the coating solution and an apple skin decreased from 21.1 to 3.7 dyne/cm by adding 1,000 ppm Tween 80; these value indicated that the apple skin was a low energy surface. Table 2 shows critical surface tension values for fruit coated with polymeric materials.

Edible solution wettability
Cerqueira et al., (2009) found that Ws values depended on the source and concentration of the galactomannans and fruit being evaluated. The following solutions were determined to be the best edible formulations for fruit: acerola-0.5% A. pavonina and 1.0% glycerol, caja-1.0% A. pavonina and 1.0% glycerol, mango and pitanga-1.5% A. pavonina and 1.0% glycerol and seriguela-0.5% C. pulcherrima and 1.5% glycerol. Lima et al., (2010) showed that the best Ws values for coating apples were obtained for coating solutions which did not contain glycerol. This result may have been due to the surface characteristics of apple, which has a high dispersive component having predominantly non-polar forces; although glycerol is polar, its presence can decrease the spreading of a solution. A formulation with 0.5% A. pavonina galactomannan, 1.5% collagen and 1.5% glycerol had the best spreading coefficient value, while an optimal solution of 0.5% C. pulcherrima and 1.5% collagen was suitable for apples. Ramírez et al., (2012) reported that apple skin had a better spreading coefficient than quince skin, most likely due to the wax which is naturally present in quince skin.
Carneiro-da-Cunha et al., (2009) discussed the effect of adding Tween 80 to a coating solution thereby lowering the surface tension of a liquid by reducing the cohesive forces and thus increasing Ws and improving compatibility between a solution and fruit surface. Tween 80 did not show any marked influence on Wa value.
Casariego et al., (2008) found that increasing chitosan and glyc-erol concentrations in coating solutions decreased the spreading coefficient and adhesion, while increasing the cohesion coeffi-cient. An inverse relationship was observed when sorbitol was used as a plasticiser. Low energy surfaces mainly interact with liquids through dispersion forces, which may explain the low work of adhesion for tomato surfaces used to characterise chitosan solutions (polar liquids), when glycerol and sorbitol were used as plasticisers.
The formulations that produced suitable wettability for straw-berries, as follows: 2% starch and 2% sorbitol, 0.3% carrageenan, 0.75% glycerol and 0.02% Tween 80 and 1% chitosan and 0.1% Tween 80 (Castro, 2005).
Skurtys et al., (2011) found that Wc decreased with increasing concentrations of Tween 20 or glycerol. The chitosan concentration affected cohesion in glycerol solutions but had a lesser effect on Tween 20 solutions. The authors found that adding glycerol to a chitosan solution decreased cranberry surface wettability by the solution. Removing wax from fruit epicarp was also found to improve wettability. Chitosan and Tween 20 solutions were found to wet the cranberry epicarp more (with or without wax) than glycerol-chitosan solutions.
Conclusions
Edible coatings can be applied to fruit to extend product shelf-life (Das et al., 2013), protect the fruit, decrease water loss, slow colour change, pH and titratable acidity during storage (Han et al., 2004) and modify the atmosphere (Park, 1999). Edible coatings are also biodegradable and help to protect the environment (Rhim et al., 2007).
Knowledge of surface properties is essential for understanding film adhesion and optimising performance characteristics, such as water permeability. Good surface wettability does not always correspond to good adhesion because wettability is necessary but not sufficient for proper adhesion (Piergiovanni and Limbo, 2010).
Different fruits' surface tension varies, depending on the particu-lar fruit texture and skin composition, such as wax coating (Ramírez et al., 2012; Skurtys et al., 2011; Velásquez et al., 2011).
References
Ali, A., Maqbool, M., Ramachandran, S., Alderson, P. G., Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit., Postharvest Biology and Technology, Vol., 58, No. 1, 2010, pp. 42-47. [ Links ]
Azeredo, H. M. C., Miranda, K. W. E., Ribeiro, H. L., Rosa, M. F., Nascimento, D. M., Nanoreinforced alginate-acerola puree coatings on acerola fruits., Journal of Food Engineering, Vol. 113, No. 4, 2012, pp. 505-510. [ Links ]
Barreiro, J., Sandoval, A., Pacheco, C. (Ed.), Operaciones de conservación de alimentos por bajas temperaturas., Venezuela, Equinoccio, 2006. [ Links ]
Busscher, H., Van Pelt, A., De Boer, P., Se Jong, H., Arends, J., The effect of surface roughening of polymers on measured contact angles of liquids., Colloids and Surface, Vol. 9, 1984, pp. 319-331. [ Links ]
Carneiro-da-Cunha, M. G., Cerqueira, M. A., Souza, B. W. S., Souza, M. P., Teixeira, J. A., Vicente, A. A., Physical properties of edible coatings and films made with a polysaccharide from Anacardium occidentale L., Journal of Food Engineering, Vol. 95, No. 3, 2009, pp. 379-385. [ Links ]
Casariego, A., Souza, B. W., Vicente, A., Teixeira, J. A., Cruz, L., Díaz, R., Chitosan coating surface properties as affected by plasticizer, surfactant and polymer concentrations in relation to the surface properties of tomato and carrot., Food Hydrocolloids, Vol. 22, No. 8, 2008, pp. 1452-1459. [ Links ]
Castro, C. M., Estudo de Estratégias para a Valorização Industrial do Morango. Tesis de Mestreado de Universidade do Minho, 2005. [ Links ]
Cerqueira, M. A., Lima, Á. M., Teixeira, J. A., Moreira, R. A., Vicente, A. A., Suitability of novel galactomannans as edible coatings for tropical fruits., Journal of Food Engineering, Vol. 94, 2009, pp. 372-378. [ Links ]
Chibowski, E., Ontiveros-Ortega, A., Perea-Carpio, R., On the interpretation of contact angle hysteresis., Journal of Adhesion Science and Technology , Vol. 16, No. 10, 2002, pp. 1367-1404. [ Links ]
Chiumarelli, M., Hubinger, M. D., Stability, solubility, mechanical and barrier properties of cassava starch - Carnauba wax edible coatings to preserve fresh-cut apples., Food Hydrocolloids, Vol. 28, No. 1, 2012, pp. 59-67. [ Links ]
Choi, W., Park, H., Ahn, D., Lee, J., Lee, C., Wettability of Chitosan Coating Solution on " Fuji " Apple Skin., Journal of Food Science, Vol. 67, No. 7, 2002, pp. 3-7. [ Links ]
Dangaran, K., Tomasula, P. M., Qi, P., Structure and Function of Protein-Based Edible Films and Coatings., In: Embuscado, M. E., Huber, K. C. (Eds.), Edilbe Films and Coatings for Food Applications., New York, Springer, 2009, pp. 25-56. [ Links ]
Das, D. K., Dutta, H., Mahanta, C. L., Development of a rice starch-based coating with antioxidant and microbe-barrier properties and study of its effect on tomatoes stored at room temperature., LWT - Food Science and Technology, Vol. 50, No. 1, 2013, pp. 272-278. [ Links ]
Drelich, J., The Significance and Magnitude of the Line Tension in Three-Phase (Solid/Liquid/Fluid) Systems., Journal of colloid and Surfaces a-Physicochemical and Engineering Aspects, Vol. 116, 1996, pp. 43-54. [ Links ]
Duan, J., Wu, R., Strik, B. C., Zhao, Y., Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions., Postharvest Biology and Technology, Vol. 59, No. 1, 2011, pp. 71-79. [ Links ]
Durango, A. M., Soares, F., Arteaga, M. R., Filmes y revestimientos comestibles como empaques activos biodegradables en la conservación de alimentos., Biotecnología en el Sector Agropecuario y Agroindustrial, Vol. 9, No. 1, 2011, pp. 112-118. [ Links ]
FAO, Prevención de pérdidas de alimentos poscosecha: Frutas, hortalizas, raíces y tubérculos., Roma, Organización de las Naciones Unidas para la Agricultura y la Alimentación, 1993. [ Links ]
Fan, Y., Xu, Y., Wang, D., Zhang, L., Sun, J., Sun, L., Zhang, B., Effect of alginate coating combined with yeast antagonist on strawberry (Fragaria X ananassa) preservation quality., Postharvest Biology and Technology, Vol. 53, 2009, pp. 84-90. [ Links ]
Gounga, M. E., Xu, S. Y., Wang, Z., Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation., Journal of Food Engineering, Vol. 83, No. 4, 2007, pp. 521-530. [ Links ]
Greiveldinger, M., Shanahan, M., A Critique of the Mathematical Coherence of Acid/Base Interfacial Free Energy Theory., Journal of colloid and interface science, Vol. 215, No. 1, 1997, pp. 170-178. [ Links ]
Guilbert, S., Gontard, N., Gorris, L., Prolongation of the Shelf-life of Perishable Food Products using Biodegradable Films and Coatings., LWT, Vol. 17, 1996, pp. 10-17. [ Links ]
Han, C., Zhao, Y., Leonard, S., Traber, M., Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria X ananassa) and raspberries (Rubus ideaus)., Postharvest Biology and Technology, Vol. 33, No. 1, 2004, pp. 67-78. [ Links ]
Han, J. H., Zhang, Y., Buffo, R. Surface chemistry of food , packaging and biopolymer materials., In: Han, J. H. (Ed.), Innovations in Food Packaging., San Diego, California, Elsevier Ltd, 2005, pp. 45-59. [ Links ]
Hernandez-Izquierdo, V., Krochta, J., Thermoplastic processing of proteins for film formation-a review., Journal of Food Science, Vol. 73, No. 2, 2008, pp. 30-39. [ Links ]
Hershko, V., Klein, E., Nussinovitch, A., Relationship between edible coatings and garlic skin., Journal of Food Science, Vol. 61, No. 4, 1996, pp. 769-777. [ Links ]
Hoover, D.,Minimally processed fruit and vegetables: Reducing microbial load by nonthermal physical treatments., Food Technology, Vol. 51, No. 6, 1997, pp. 66-71. [ Links ]
Janczuk, B., Bialopiotrowicz, T., Surface free-energy components of liquids and low energy solids and contact angles., Journal of Colloid and Interface Science, Vol. 127, No. 1, 1989. pp. 189-204. [ Links ]
Kader, A. A., Postharvest Biology and Technology: An Overview., In: A. A. Kader, (ed.), Postharvest Technology of Horticultural Crops, Third Ed, California, University of California Agriculture and Natural Resources, 2002. [ Links ]
Kaelble, D. H., Cirlin, E. H., Dispersion and polar contributions to surface tension of poly(methylene oxide) and Na-treated polytetrafluoroethylene., Journal of Polymer Science, Vol. 9, No. 2, 1971, pp. 363-368. [ Links ]
Kester, J., Fennema, O., Edible films and coatings: A review., Food Technology, Vol. 40, No. 12, 1986, pp. 47-59. [ Links ]
Kiely, L. J., Olson, N. F., The physicochemical surface characteristics of Lactobacillus casei., Food Microbiology, Vol. 17, No. 3, 2000, pp. 277-291. [ Links ]
Krochta, J., Mulder-Johnston, C., Edible and biodegradable polymer films: challenges and opportunities., Food Technology, Vol. 51, 1997, pp. 61-74. [ Links ]
Letien, C., Vachion, C., Mateescu, M., Lacroix, M., Milk protein coatings prevent oxidative browning of apples and potatoes., Journal of Food Science, Vol. 66, 2001, pp. 512-516. [ Links ]
Lima, Á. M., Cerqueira, M. A., Souza, B. W. S., Santos, E. C. M., Teixeira, J. A., Moreira, R. A., Vicente, A. A., New edible coatings composed of galactomannans and collagen blends to improve the postharvest quality of fruits - Influence on fruits gas transfer rate., Journal of Food Engineering, Vol. 97, No. 1, 2010, pp. 101-109. [ Links ]
Lin, D., Zhao, Y., Innovations in development and application of edible coatings for fresh and minimally processed fruits and vegetables., Comprehensive Reviews in Food Science and Food Safety, Vol. 6, 2007, pp. 1-15. [ Links ]
Lu, J., Zhang, H., Wei, D., Hu, Y., A Method for Determining Surface Free Energy of Bamboo Fiber Materials by Applying Fowkes Theory and Using Computer Aided Machine Vision Based Measurement Technique., Journal Shanghai Jiaotong University (Science), Vol. 17, No. 5, 2012, pp. 593-597. [ Links ]
Martínez-Castellanos, G., Uso de bacterias lácticas en recubrimientos de quitosano para la conservación poscosecha de litchi y rambután., Tesis de doctorado presentado a la Universidad Autónoma Metropolitana, Unidad Iztapalapa, 2009. [ Links ]
Marzo-Rojas, I., Efecto del tipo y contenido de aceites esenciales sobre las propiedades mecánicas y barrera de películas comestibles basadas en zeína., Trabajo fin de carrera presentado a la Universidad Pública de Navarra, 2010. [ Links ]
Mishra, V. K., Gamage, T. V., Postharvest Physiology of Fruit and Vegetables., In: M. S. Rahman (Ed.), Handbook of Food Preservation., 2nd ed., Boca Raton, 2007, pp. 19-48. [ Links ]
Oliva, G., Barbosa-Cánovas, G., Edible coatings for fresh-cut Fruits., Critical Reviews in Food Science and Nutrition, Vol. 45, 2005, pp. 657-670. [ Links ]
Palacios Martínez, L., Caracterización estructural y superficial de membranas microporosas., Tesis de Doctorado presentado a la Universidad de Valladolid, 1999. [ Links ]
Park, H., Development of advanced edible coatings for fruits. Trends in Food Science and Technology, Vol. 10, 1999, pp. 254-260. [ Links ]
Pavlath, A. E., Orts, W., Edible Films and Coatings: Why, What, and How?, In: Embuscado, M. E., Huber, K. (Ed.), Edilbe Films and Coatings for Food Applications, New York, Springer, 2009. [ Links ]
Piergiovanni, L., Limbo, S., Food packaging Materiali, tecnologie e qualità degli alimenti., Italia, Springer-Verlag Italia, 2010, pp. 562. [ Links ]
Qi, H., Hu, W., Jiang, A., Tian, M., Li, Y., Extending shelf-life of Fresh-cut "Fuji" apples with chitosan-coatings., Innovative Food Science , Emerging Technologies, Vol. 12, No. 1, 2011, pp. 62-66. [ Links ]
Quintero, C. J., Falguera, V., Muñoz, H. A., Películas y recubrimientos comestibles: importancia y tendencias recientes en la cadena hortofrutícola. (Edible films and coatings: importance and recent trends in the horticultural chain.), Tumbaga, Vol. 5, 2010, pp. 93-118. [ Links ]
Ramos, Ó. L., Reinas, I., Silva, S. I., Fernandes, J. C., Cerqueira, M. A., Pereira, R. N., Malcata, F. X., Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom., Food Hydrocolloids, Vol. 30, No. 1, 2013, pp. 110-122. [ Links ]
Ramírez, C., Gallegos, I., Ihl, M., Bifani, V., Study of contact angle, wettability and water vapor permeability in carboxymethylcellulose (CMC) based film with murta leaves (Ugni molinae Turcz) extract., Journal of Food Engineering, Vol. 109, No. 3, 2012, pp. 424-429. [ Links ]
Rhim, J. W., Leeb, J. H., Ng, P. K. W., Mechanical and barrier properties of biodegradable soy protein isolate-based films coated with polylactic acid., LWT - Food Science and Technology, Vol. 40, 2007, pp. 232-238. [ Links ]
Ribeiro, C., Vicente, A. A., Teixeira, J. A., Miranda, C., Optimization of edible coating composition to retard strawberry fruit senescence., Postharvest Biology and Technology, Vol. 44. No. 1, 2007, pp. 63-70. [ Links ]
Robles-Sánchez, R. M., Rojas-Graü, M. A., Odriozola-Serrano, I., González-Aguilar, G., Martin-Belloso, O., Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes., LWT - Food Science and Technology, Vol. 50, No. 1, 2013, pp. 240-246. [ Links ]
Rudawska, A., Jacniacka, E., Analysis for determining surface free energy uncertainty by the Owen-Wendt method., International Journal of Adhesion, Adhesives, Vol. 29, 2009, pp. 451-457. [ Links ]
Rulon, J., Robert, H., Wetting of low-energy surfaces., In: J. Berg (Ed.), Wettability, Marcel Dekker Inc., 1995, pp. 4-73. [ Links ]
Skurtys, O., Acevedo, C., Pedreschi, F., Enrione, J., Osorio, F., Aguilera, J. M., Food Hydrocolloid Edible Films and Coatings., In: E. Strain (Ed.), Food Hydrocolloids: Characteristics, Properties, Nova Science Publishers, Inc., 2010. [ Links ]
Skurtys, O., Velásquez, P., Henriquez, O., Matiacevich, S., Enrione, J., , Osorio, F., Wetting behavior of chitosan solutions on blueberry epicarp with or without epicuticular waxes., LWT - Food Science and Technology, Vol. 44, No. 6, 2011, pp. 1449-1457. [ Links ]
Song, B., Springer, J., Determination of Interfacial Tension from the Profile of a Pendant Drop Using Computer-Aided Image Processing., Journal of colloid and interface science, Vol. 184, No. 1, 1996, pp. 77-91. [ Links ]
Souza, B. W. S., Cerqueira, M. A., Teixeira, J. A., Vicente, A. A., The Use of Electric Fields for Edible Coatings and Films Development and Production: A Review., Food Engineering Reviews, Vol. 2, No. 4, 2010, pp. 244-255. [ Links ]
Tracton, A. A., Coatings Technology Handbook, 3rd ed., U. S. A. Taylor, Francis., 2005. [ Links ]
Tzoumaki, M. V., Biliaderis, C. G., Vasilakakis, M., Impact of edible coatings and packaging on quality of white asparagus (Asparagus officinalis, L.) during cold storage., Food Chemistry, Vol. 117, No. 1, 2009, pp. 55-63. [ Links ]
Ulloa, J. A., Frutas auto estabilizadas en el envase por la tecnología de obstáculos., 1st ed., México, Universidad Autónoma de Nayarit, 2007. [ Links ]
Vachon, C., D'Aprano, G., Lacroix, M., Letendre, M., Effect of edible coating process and irradiation treatment of Strawberry Fragaria spp. on storage - keeping quality., Journal of Food Science, Vol. 68, 2003, pp. 608-612. [ Links ]
Vargas, M., Albors, A., Chiralt, A., Gonz, C., Quality of cold-stored strawberries as affected by chitosan - oleic acid edible coatings., Postharvest Biology and Technology, Vol. 41, 2006, pp. 164-171. [ Links ]
Velásquez, P., Skurtys, O., Enrione, J., Osorio, F., Evaluation of Surface Free Energy of Various Fruit Epicarps Using Acid-Base and Zisman Approaches., Food Biophysics, Vol. 6, No. 3, 2011, pp. 349-358. [ Links ]
Viña, S. Z., Mugridge, A., García, M. A., Ferreyra, R. M., Martino, M. N., Chaves, A. R., Zaritzky, N. E., Effects of polyvinylchloride films and edible starch coatings on quality aspects of refrigerated Brussels sprouts., Food Chemistry, Vol. 103, No. 3, 2007, pp. 701-709. [ Links ]
Zisman, W., Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution., Advances in Chemistry, Vol. 43, Washington, DC, Fowkes, 1964, pp. 1-51. [ Links ]
Zouvelou, N., Mantzouris, X., Nikolopoulos, P., Interfacial energies in oxide/liquid metal systems with limited solubility., Journal of Adhesion and Adhesives, Vol. 27, No. 5, 2007, pp. 380-386. [ Links ]
Zenkiewicz, M., Comparative study on the surface free energy of a solid calculated by different methods., Polymer Testing, Vol. 26, 2007, pp. 14-19. [ Links ]













