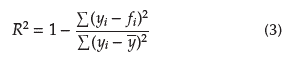Introduction
Arracacha roots (AR) or creole celery (Arracacia xanthorrhiza Bancroft.), are a tuber with high nutritional value that offers large amounts of minerals and carbohydrates compared to cassava (Londono-Restrepo, Rincon-Londono, Contreras-Padilla, Millan-Malo, and Rodriguez-Garcia, 2018; Otache, Ubwa, and Godwin, 2017). Furthermore, Colombia is currently the world's largest producer of arracacha with an annual production of 855 840 t/year in 2018 (MinAgricultura and Agronet, 2018).
Moreover, an arracacha crops are characterized by having productive cycles throughout the year, and their quality is definitely affected by pre-harvest, harvest, and post-harvest processes. Poor implementations stemming from cultural habits influence arracacha in terms of sprout decomposition, production heterogeneity, and the frequency of crop diseases (Rana and Kumar, 2017), thus generating a highervolume of non-quantified waste in post-harvest, as well as creating low production yields, poor product quality, and, in turn, environmental emissions problems such as nitrous oxide, carbon monoxide, among others; these compounds contribute to the greenhouse effect (Galford et al., 2020; D. Huang et al., 2019).
Likewise, the use of these agricultural residues is quite extensive and highly studied, including the extraction of compounds of pharmaceutical interest (Didaskalou, Buyuktiryaki, Kecili, Fonte, and Szekely, 2017), fertilizers (Lupton, 2017), and fermentable sugars for the production of biofuels (C. Huang, Jeuck, and Yong, 2017; Nair, Lennartsson, and Taherzadeh, 2017).
Obtaining biofuel from vegetable waste is an alternative to solve issues with oil dependency that come from the 1970s energy crisis, where the fossil fuel supply decreased considerably, and the ensuing riots created the necessity to contribute to environmental management (Venn, 2016).
Even though there was a collapse in crude oil prices, which fell to negative values due to the current COVID-19 pandemic (Aloui, Goutte, Guesmi, and Hchaichi, 2020), there is still an interest in modifying oil-powered engines. This has led to research on the topic of ethanol as a fuel source for the automotive, energetic and agroindustry industry since the mid-nineteenth century until now (Awad et al., 2018).
Bioethanol increases gasoline octane, improving its combustion and efficiency in the conversion to mechanical energy. At the same time, it reduces fuel consumption by means of a higher release of free radicals (H, OH, and O) and reducing emissions of hydrocarbons (HC), carbon monoxide (CO), and nitrogen oxides (NOx) (Aditiya, Mahlia, Chong, Nur, and Sebayang, 2016; Awad et al., 2018). This complies with Colombian law nr. 693 of 2001 (Colombian Congress, 2001), which states that the automotive industry has to contain oxygenated components such as fuel alcohols, with the purpose of controlling air pollution.
Concerning the above, the ethanol production process is developed in four steps: pretreatment, fermentation, separation, and post-treatment of the liquid fraction (Zabed, Sahu, Suely, Boyce, and Faruq, 2017). As for pretreatments, benefits depend on the kind of material. They could be physical (increasing the surface area and pore volume) or chemical (alkali and acid). Their function is to decrease the degree of cellulose polymerization and its crystallinity, thus allowing for a major production of reducing sugars (Sarkar, Ghosh, Bannerjee, and Aikat, 2012). However, there are some significant disadvantages in the use of physical methods, such as high energy consumption, which is environmentally unfriendly and non-viable for a commercial process.
Chemical pretreatments have the presence of inhibitors in each of the mentioned stages (Zabed et al., 2017), which causes the delay of yeast activity and an extension of fermentation time. Due to hydrolytic pretreatments applied to vegetable material (like furfural, hydroxymethylfurfural, and acetic acid, among other compounds) (Maiti et al., 2018) it has a major negative effect on enzymes (Wojtusik, Villar, Zurita, Ladero, and Garcia-Ochoa, 2017).
In this context, the purpose of this paper is to study the hydrolysis of the carbohydrates present in the post-harvest agricultural discards of arracacha (unsalable roots) to obtain fermentable sugars, assessing inhibiting compounds (acetic and furfural acid), despite AR having phenolic compounds (Leja et al., 2013), which are major enzyme and microbial inhibitors that can potentially affect the production of ethanol during fermentation.
Methodology
Sample conditioning
AR samples were purchased from a crop in Cajamarca, Tolima, Colombia. Only roots that were not marketable, malformed, or with mechanical or pest damage were used in the tests. Samples were dried to an average humidity of 13% in a convection oven (60 °C, 72 h), and then the particle size was reduced to 1 mm using a hammermill.
Analytical techniques
The contents of moisture (method 925.10), crude protein (method 955.04), ether extract (method 963.15), crude fiber (method 962.09), and ash (method 941.12) were determined for each residue by means of the official methodology (AOAC, 2000). Also, neutral detergent fiber (NDF), acid detergent fiber (ADF), cellulose, hemicellulose, and lignin were all quantified according to the Van Soest methodologies (P. Van Soest and Wine, 1968; P. J. Van Soest, 1963).
Total and reducing carbohydrate quantification were performed by extraction with absolute ethanol in dilution 1:3 (w/v), filtered on quantitative paper and refrigerated until use. The quantification of both total carbohydrates (TC) and reducing sugars was carried out using the Antrona (Leyva et al., 2008) and DNS (Miller, 1959) spectrophotometric methods using a UV-Vis Helios Gamma spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 620 nm and 540 nm, respectively.
Physicochemical pretreatment
The AR was submitted to pressures of 0,1034 MPa (100,55 °C), 0,2068 MPa (121,28 °C), and 0,4137 MPa (144,83 °C) (saturated steam), and it diluted in distilled water to a ratio of 1:15 for one hour. The steam supply was generated in a 2-inch vertical, pirotubular boiler with a capacity of 2 BHP (Equipos y Calderas Industriales E U, Bogotá) (Carranza Saavedra, Alvarado Núñez, Méndez Reyes, Valenzuela Real, and Solanilla Duque, 2015).
Chemical pretreatment
The samples were hydrolyzed with sulfuric acid (H2SO4) at concentrations of 0,25, 0,50, 1,00, and 2,00 M in 1:15 dilution at a temperature of 60 °C, refluxing at the atmospheric pressure of Ibagué, in the department of Tolima, Colombia (Carranza Saavedra et al., 2015).
Residence time
The resistance time for sugar splitting in every case was performed during 1 h. Then, in order to observe the effect of residence time on the samples, the best pretreatment (physical or chemical) was selected, and the residence time was increased to 2 h of hydrolysis. Next, the contents of total and reducing carbohydrates were analyzed.
Enzymatic pretreatment
From the best pretreatment, the samples were submitted to enzymatic hydrolysis to increase their reducing carbohydrates (RC) with Novozymes' Liquozyme SC DS a-amylase at 1,5, 5, and 10 kilo Novo units (KNU)/g (one KNU was defined as the amount of enzyme that hydrolyzed 5,26 g of starch (soluble starch) per hour under Novozymes's standard conditions for a-amylase determination) with diacid phosphate buffer pH 6 at temperature of 80 °C and 2 h with constant agitation (150 rpm).
Samples were taken every 30 minutes. The enzyme was inactivated at 95 °C for 5 min, and the samples were later centrifuged at 5 000 rpm for 10 min, determining RC immediately (Rathore, Paulsen, Vidal Jr., and Singh, 2009). The rate constant for the formation of first-order fermentable sugars was also evaluated, which is directly proportional to the concentration of the substrate and can be evaluated at different concentrations of enzyme. It is described using Equation (1):
Where, rc is the concentration of RC, k is the first order kinetic constant, and t is time. Additionally, the solution of the linearized Equation (1), where k is the slope, is described using Equation (2):
Where rc0 is the concentration of initial RC.
Inhibiting Compounds in Pretreated Liquid Fractions
Determination of acetic acid content: Acetic acid was quantified through titratable acidity (Cheng et al., 2008). The results are expressed as equivalent milligrams of acetic acid per milliliter (mgEAA/mL).
Furfural quantitative determination: Furfural quantification was implemented according to the methodology described by the AOAC (2000). 25 mL of pretreatment liquid were taken to be distilled through a fractionating column. The fraction was collected at boiling temperature (161,20 °C). Later, 1 mL of the distilled result was taken and gauged with alcohol (99,80%) to complete 10 mL. Sample absorbance, alcohol white, and the standard (furfural) 277 nm were determined in a spectrophotometer. The results are expressed as equivalent furfural milligrams per milliliter (FEmg/mL).
Statistical Analysis
The results obtained in the study were expressed as the mean and the standard deviation of three determinations for each pretreatment. Analysis of Variance (ANOVA) and Multivariate Analysis of Variance (MANOVA) at a 95% confidence level were used to compare the mean values of each determination among the different pretreatments.
The STATGRAPHICS Centurion XV statistical package was used for the analysis of the obtained results. The treatments were executed through randomized complete block design (RCBD) and averaged differences using the least significant difference (LSD) for each one. The coefficient of determination (Equation 3) was used to assess the goodness of fit of the linearization of Equation (1).
Where f i , y i , and y are the model data, experimental data, and the mean of the experimental data, respectively.
Results and Discussion
Bromatological and carbohydrates analysis
Table 1 shows the results of the bromatological analysis, as well as the composition of cell walls in AR. The protein content in the studied samples was higher than in other types of arracacha (Londoño-Restrepo et al., 2018), and did not exceed the range between 4,00% and 8,00%. When added to the TC content, it represents a significant amount of calories that partly support the conversion of energy in animals that are fed with these residues.
The ethereal extract found to have the greatest amount was the yellow and purple arracacha plant (Londoño-Restrepo et al., 2018; Palacios, Morales, and Arias, 2011), with documented fat values of 0,90% (dry basis).
The crude fiber content was almost ten times higher than in other studies (Londoño-Restrepo et al., 2018; Palacios et al., 2011), which shows a promising composition for the extraction of RC. The ash contents had similar values as the reported cases of arracacha flour, which means it is an important source of minerals in use for human and animal food.
Table 1 shows the values of the study of the cell wall (ADF, NDF, hemicellulose, cellulose, and lignin) which evinces a small amount of lignocellulosic material in its structure, as well as the fact that, according to studies, it contains between 75% and 90% starch on dry basis (Londoño-Restrepo et al., 2018; Palacios et al., 2011). Therefore, it was not necessary to carry out tests with sodium hydroxide. It was also observed that it did not contain detectable lignin in its structure.
Table 1 Physicochemical analysis of AR

Results are the mean of three determinations (n = 3), ± represent standard deviation. Similar lower-case letters indicate that there is no statistical difference between treatments (p < 0,05).
Source: Authors
The carbohydrates in unprocessed AR (control) were characterized in order to evaluate the increase or decrease in present sugars during the pretreatments. The studies reported 27,39% and 1,63% of total and reducing carbohydrates, respectively, a considerable amount compared to cane cachaza, penelera cane chuff, rice shell, Tahití lime (citrus latifolia), and common lemon (citrus aurantifolia), which are reported as feedstock to obtain ethanol, especially by way of fermentation (Sánchez-Riaño, Barrero, and Murillo, 2010).
Pretreatments
Physicochemical pretreatment:Figure 1 shows the different pressures at which the AR were treated to obtain reductive carbohydrates at different temperatures. The MANOVA reported significant differences (p < 0,05) between the total and reducing carbohydrates by physicochemical treatment with a 95% level of significance.

Source: Authors
Figure 1 Total and reducing carbohydrate contents of arracacha obtained at different pressures with saturated steam. Vertical bars represent standard deviation. Similar lower-case letters indicate that there is no statistical difference between treatments (p < 0,05).
Now, elevating the temperature of the starchy material proved its sensitiveness, which showed an increase in the quantification of TC with a significant pressure differences (p < 0,05) between 0,10 MPa (0,52 ± 0,03 g/g), 0,20 MPa (0,53 ± 0,01 g/g), and 0,41 MPa (0,67 ± 0,05 g/g) with respect to the control sample. However, the pressure at 0,41 MPa allows an upper splitting of high molecular weight carbohydrates, attributed to hydrolyzed starch by the action of water molecules, which speed up the absorption at high temperatures, causing the swelling of the starch and breaking the granule; releasing the amylose and amylopectin molecules; and later, breaking their glycosidic bonds (Kong et al., 2016).
On the other hand, the variability of the results in terms of RC is shown in Figure 1, indicating that there are significant differences (p < 0,05) in the RC obtained at 0,41 psi in comparison with the control sample. Moreover, the simple sugars do not exceed 0,10 ± 0,01 g/g because of amylose and amylopectin molecules are released only in the rupture of starch granule, causing the breakup of 1,4 and 1,6 glycosidic bonds, respectively, but in small proportions (Shigematsu et al., 2017).
Chemical pretreatment:Figure 2 shows the sugar content (total and reducing) of arracacha when submitted to chemical hydrolysis. There seems to be a proportional or consequent behavior with the increase in acid concentration during the pretreatment. However, the 2,0 M acid concentration had an observed decline of total and reducing carbohydrates with a significant statistical effect (p < 0,05). TC reached their highest hydrolysis point at 1,0 M (0,73 ± 0,03 g/g). A similar behavior was observed in RC at an acid concentration of 1,0 M with a 0,33 ± 0,03 g/g quantification. This phenomenon could be due to the degradation of amylose chains, and even amylopectin chains by H2SO4, which, as a consequence, releases total and reducing sugars (Li et al., 2020).

Source: Authors
Figure 2 Total and reducing carbohydrate contents of arracacha obtained at different concentrations of H2SO4. Vertical bars represent standard deviation. Similar lower-case letters indicate that there is no statistical difference between treatments (p < 0,05).
Furthermore, the increase of H2SO4 concentration for the hydrolysis of arracacha samples was harmful, because carbohydrates began to degrade, turning into unwanted compounds for fermentation (phenols and furfurals) (Guerrero, Ballesteros, and Ballesteros, 2017).
Increase in residence time: In pretreatments (physicochemical and chemical), it was evident that hydrolysis with H2SO4 at a 1,0 M concentration is the most effective method to obtain RC from arracacha discards.
Judging from the assessment of the residence time, higher yields were obtained at two hours with 0,52 ± 0,09 g/g (RC) compared to the one-hour treatment. This confirms a decrease in TC (0,56 ± 0,1 g/g), possibly due to the rupture of the hydroxyl groups present in starchy materials (Peñaranda Contreras, Perilla Perilla, and Algecira Enciso, 2008).
Enzymatic pretreatment: The enzymatic hydrolysis for arracacha residues was implemented with α-amylase after choosing the best acid hydrolysis pretreatment (H2SO4 1,0 M, 2 h). Figure 3A shows the enzyme kinetics when it reacts with the substrate. An ANOVA revealed a statistic interaction (p < 0,05) with a 95,0% level of confidence between the fermentable sugar concentrations present in the hydrolyzed sample (g/g) and the enzyme concentration, showing a considerable increase in the monomeric sugars, due to the action that the enzyme exerts along any point of the carbohydrate chain (1-4 glucosidic bonds), hydrolyzing them in dextrin from amylose (Oliveira, Pinheiro, Fonseca, Cabrita, and Maia, 2019).
Therefore, the maximum conversion from substratum to dextrose is obtained within 2 hours, and in absence of a significant difference, according to Fisher's LSD method, between 1,5 and 5,0 KNU/g of concentration of the a-amylase enzyme, it is understood that 1,5 KNU/g is the concentration at which the enzyme works better, and the best results from enzymatic hydrolysis (0,56 ± 0,05 g/g) are obtained. Also, the highest concentration of the α-amylase enzyme might not have been attained because it could have been inhibited by the relation between enzyme and substrate, decreasing the hydrolysis speed of starch to glucose (Zhang et al., 2020).
Low values were obtained for corn starch hydrolysate with a reducing sugar content of 12-14% with the same enzyme and operation time, but without pretreatment (Z. Li, Wang, and Shi, 2019). Thus, it is possible that the enzyme conversion with pretreatment could find suitable applications in productions of sugars and chemicals made by the fermentation of sugars.
Alternatively, it is important to characterize the reaction kinetics for a reducing sugar gain. Contents of RC increase as part of the aging process, as shown in Figure 3A. First-order kinetics correctly described an increase in reducing sugars (R2 > 0,95, 1,5 KNU/g in Equation (1)), as shown Figure 3B.

Source: Authors
Figure 3 Hydrolysis of carbohydrates in AR with α-amylase enzyme. A: Enzymatic kinetics of reducing carbohydrate B: Kinetic analysis of the reducing sugar content. Vertical bars represent standard deviation. Lines in A are a guide for the eye and in B represent first-order fitting according to Equation 1.
Chemical compounds react and convert to other species, and the rate constant indicates the speed of the change in concentration. The half-life time of each chemical species is an indicator of the time that a given compound increases its concentration byafactor of0,02(k) to 1,5 (NKU/g). Therefore, it is a standardized parameter that indicates the time at which a compound is degraded to simple sugars.
Evaluation of inhibiting agents
The assessment of the inhibiting compounds present in starchy material was made from AR hydrolysates. It was then concluded that this is the most efficient method to obtain reducing sugars using the chemical pretreatment with H2SO4 at 1,0 M and 2 h of residence time. In the hydrolyzed samples, an acetic acid formation was observed, due to the conditions of the chemical pretreatment applied to the roots (3,12 ± 0,11 mgEAA/mL). This result is associated with presence of acetyl groups by deacetylation of hemicelluloses during the pretreatment. These are not degraded despite the prolonged storage time, and they were possibly entirely hydrolyzed to form acetic acid by the action of the acid environment in which the pretreatment was carried out (Maiti et al., 2018), regardless of the residence time at which it was submitted.
There was also an effect (0,28 ± 0,002 mgEF/mL) of H2SO4 concentration, temperature, and residence time in the formation of the environment that fosters pentose dehydration, until their degradation to furfural in pre-treated starchy waste. This could be explained by the way in which manymonomeric sugars (pentoses)were possiblyhydrolyzed during the process of acid pretreatment in AR; these sugars would be degraded to this inhibiting compound (furfural) (Maiti et al., 2018). Lastly, AR have phenolic compounds within their structure, which act well as major enzymes and microbial inhibitors.
Conclusions
The physicochemical pretreatment with distilled water under high pressures turns out to be a soft method of carbohydrate hydrolysis compared to chemical pre-treatment in H2SO4 at 1,0 M with quantification of 51,8% of RC by dry sample of roots and a residence time of 2 h.
A further pretreatment with a-amylase increases the fermentable sugar yield with arracacha discards, reaching a conversion of 1,04 g of fermentable sugars per gram of dry vegetable material.
The fermentation of non-detoxified hydrolysates is characterized by a limited productivity and yield, due to the presence of a variety of compounds that act as potential inhibitors of the cellular metabolism, such as furfural and acetic acid found in AR after the hydrolysis with diluted H2SO4. Therefore, the use of mechanisms that remove most of these toxic compounds in the hydrolysates is necessary, thus improving the metabolism of the microorganisms responsible for starchy material fermentation.
Furthermore, the identification of the RC type that is generated in the hydrolysis is used to establish the appropriate biological mechanism for fermenting ethanol, considering the detoxification tests before the fermentation.
This study of carbohydrate hydrolysis in non-marketable food significantly shows the ease of taking advantage of agricultural waste and converting it into clean energy, thereby contributing to the continuous development of producing fermentable sugars as a workhorse in the production of bioethanol.