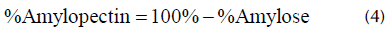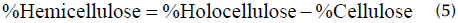Introduction
Plastics are a family of materials made from fossil resources and present in most sectors of the economy. Their high consumption is causing environmental problems such as prolonged permanence in landfills, greenhouse gas emissions during burning, and irreversible damage to marine ecosystems. Approximately eight million tons (Mt) of plastic reach the sea annually, with consequences from the surface to the seabed (Parker, 2018).
These materials enter the aquatic, atmospheric, and terrestrial systems, migrate among themselves, accumulate in the environment, and pass to humans through the food chain (Li et al., 2020). Plastics are estimated to be responsible for emitting 400 Mt of greenhouse gases each year. If the current production rate continues, this industry alone will consume 20% of the world's oil production by 2050 (Mahapatra et al., 2020). Hence the need to obtain biomaterials from plant or animal biomass that, if possible, do not compete with the food needs of the human population. One of them is bioplastics, which can be biologically based, biodegradable in nature, or with both characteristics.
Agribusiness waste is a source of plant biomass from which a variety of products are obtained through chemical or microbiological treatments (Gutiérrez-Macías et al., 2017). Among them are those generated during the production and processing of plantain, where leaves, pseudostems, rachis, peels, and fruits are identified as residues (Granda et al., 2005; Mondragón-García et al., 2018). This constitutes between 20 and 30% of the available biomass used in banana harvesting (Belalcázar and Sylvio, 1991). During processing, the peel is discarded, which constitutes 30% of the weight of the fruit (Wadhwa and Bakshi, 2013).
The Ecuadorian economy has a strong agricultural participation. Among its main products are bananas, with a production of almost 750 thousand metric tons for 2019 (Ministerio de Agricultura y Ganadería, 2020). The destination of this fruit is exportation, local consumption, and processing for the production of snacks, banana flour, and vacuum-packed peeled bananas in small and medium-sized industries. The waste generated in this activity contains compounds of interest such as cellulose, hemicellulose, and starch, which is useful in the production of bioplastics.
In the Central and Latin American contexts, the possibility of applying biorefinery processes to use banana residues as raw material for the production of biofuels, nanocellulose fibers, bioplastics, and other high-value products has been evaluated (Redondo-Gómez et al., 2020). Some research works claim to have obtained biopolymers from banana peel and corn starch, banana peel, and glycerol (Kader-Sultan and Wan-Johari, 2017; Rusdi et al., 2020). In this vein, bioplastic has been made from banana peel, the pseudostem of the plant, and plasticizers. The objective of this research was to evaluate the impact that the plasticizer type has on the physical and mechanical properties of bioplastic.
Experimentation
The employed banana peels and pseudostems came from plantations and artisan factories located in the province of Manabí-Ecuador. The procedures for extracting and characterizing the residue, preparing the thermoplastic mixture, and determining its properties are described below.
Materials extraction and characterization
The flour was extracted from the banana peel via dry grinding. To this effect, twelve healthy immature bananas were selected, washed with drinking water, and disinfected by immersion in a 1% (w/v) NaClO solution for 10 min. The endocarp was removed and dehydrated in a hot air tray dryer at 40 °C for 12 h. It was ground and sieved twice on 40 and 20 pm sieves. The flour was packed in polyethylene bags until use (Mazzeo and Alzate, 2008).
The banana peel flour was characterized in terms of moisture, starch content, gelatinization temperature, water absorption index (WAI), water solubility index (WSI), swelling power (SP), amylose, and amylopectin content. The moisture was determined with a BMA 150 thermobalance (Tirado et al., 2015). The presence of starch was confirmed by means of a colorimetric analysis, applying an iodine solution stain on the sample (López et al., 2014).
The gelatinization temperature was determined by dissolving 10 g of flour on a dry basis (DB) in 100 mL of distilled water. 50 mL of the suspension were taken and immersed in a thermostatic bath at 85 °C with stirring. As the paste formed, the temperature was recorded with a thermometer until it stabilized (Grace, 1977).
The procedure for determining WAI, WSI, and SP can be described as follows. 1,25 g of flour were taken, placed in a centrifuge tube with 30 mL of distilled water at 60 °C, and stirred. The mixture was placed in a thermostatic bath at 60 °C for 30 min, stirring after the first 10 min of heating. It was centrifuged at room temperature at 4 900 RPM for 30 min. The volume (V) was measured. 10 mL of the supernatant were taken and dried in an oven at 70 °C for 14 h. Centrifuge tubes weighed the sediment and dry residue (Anderson, 1982). The calculations were made by means of Equations (1), (2), and (3).
Amylose was quantified by spectrophotometry with a wavelength of 620 nm, using the calibration curve for the standard solution (Galicia et al., 2012). The amylopectin content was determined by difference using Equation (4).
Cellulose was extracted from the pseudostem of the banana. To this effect, the fruit was cut into sections, washed with distilled water, separated by layers, and washed again. It was dried to constant weight and degreased using Soxhlet extraction. It was submitted to an acid treatment with a solution of CH3(COOH) at 80% (w/v) and HNO3 at 65% (w/v) with stirring. Then, it was shaken with a 10% (w/v) NaOH solution, washed, and dried at 55 °C. The obtained pulp was ground and sieved to reduce its size (Romero-Viloria et al., 2014). The cellulose content was determined with the Kurschner and Hoffer method, holocellulose by applying the TAPPI T-21 method, lignin with the Klason method, and hemicellulose through Equation (5) (Romero- Uscanga et al., 2014).
Preparation of the thermoplastic mixture
Before defining the experimental design, a preliminary test of 16 formulations was carried out with different compositions of banana peel flour, cellulose, water, glycerin, sorbitol, and 5 and 15% NaOH solutions (w/v) in order to identify the mixtures that form the film. In each plastic mixture, the pH was measured with a potentiometer. Based on the results, a mixture design was elaborated, with fixed amounts of banana peel flour (5 g), 15% NaOH (5 mL), and water (4 mL), varying the concentrations of the plasticizers used, which were glycerol and sorbitol (0 to 2 g) (Table 1).
Table 1 Design of mixtures for experimentation
| Formulation | Sorbitol (%) | Glycerol (%) |
| F1 | 100 | 0 |
| F2 | 75 | 25 |
| F3 | 50 | 50 |
| F4 | 25 | 75 |
| F5 | 0 | 100 |
Source: Authors
The procedure consisted of mixing the banana peel flour with the water and the NaOH solution at 20 °C. The mix was stirred at 400 RPM for 10 min and placed in a thermostatic bath. Then, the selected plasticizer was added, and the temperature was increased to 65 °C with constant stirring. The obtained gel was cooled to 20 °C in order to eliminate bubbles, and it was deposited on Petri dishes to form the plastic films via the casting method. It was dried at room temperature for 24 h and then demolded. After determining the characteristics of the obtained bioplastic, the formulations with the best physical appearance and resistance were selected. 0,50 g of cellulose were added to the selected formulations, and then the effect caused by this filler material on the physical, chemical, and mechanical properties of the material was determined.
Bioplastic properties
The following characteristics were evaluated in the obtained film: thickness, water vapor permeability (WVP), biodegradability, and tension force at the break. The thicknesses were measured directly on the film in three different sections with a precision 25 x 1 mm linear vernier C4.svg micrometer (Anchundia et al., 2016). The WVP was measured according to the modified E96-80 ASTM standard as described by Joaqui and Villada-Castillo (2013), for which samples of the material were sectioned into 2 x 2 cm pieces and used to seal a hole at the top of a 1 x 1 cm plastic cuvette containing distilled water. The cuvette was placed in a desiccator for 24 h. The initial and final weights of the film were determined, as well as the moisture percentage. To calculate the WVP, Equations (6) and (7) were used.
where WVP is the water vapor permeability (g/Pa.sm), and WVTR is the water vapor transmission rate (g/s.m2) calculated from the mass m (g), the time t (s) corresponding to the duration of the experiments (24 h), and the area A of the film exposed to the transfer of humidity (m2). T is the average thickness of the film (m), Pw is the partial pressure of pure water vapor (3 160 Pa at 25 °C), and ARH is the relative humidity gradient between the container (0%) and the medium (41%).
Biodegradability was measured through the weight loss of the thermoplastic material, based on a soil burial test (Saffian et al., 2016). The natural soil was collected free of composting material. The samples were weighed, placed with soil in polyethylene bags, and kept at room temperature with a relative humidity of approximately 43% for 40 days. Every ten days, the samples were removed, rinsed with water to remove soil residues, dried for one hour at room temperature to remove moisture, and finally weighed. The biodegradability percentage of the bioplastic for the evaluated environment was determined via weight difference with Equation (8).
Tensile tests were carried out to measure the tensile strength and break time of the film. To this effect, a Nestor universal machine was used according to the method described in the modified ASTM D-882 standard (ASTM International, 2018). The diameter, area, and thickness of three samples of the films were measured. They were then placed between the clamping presses of the equipment and subjected to tension until they broke.
Results and discussion
The colorimetric determination of the banana peel flour confirmed the presence of starch because it took a blue color when in contact with iodine. The rest of the results obtained from the analysis carried out for the characterization in terms of humidity, gelatinization temperature, WAI, WSI, SP, amylose, and amylopectin, is presented in Table 2.
The moisture in the banana peel flour was 14,80 + 0,40, higher than that reported in similar studies for banana flour and starch, respectively, which reported values of 9,26 and 9,45% (Montoya et al., 2014) and 8 and 9,27% (Pelissari et al., 2012). This variation in the moisture content of the flour could be affected by the environmental conditions of the laboratory where the analysis was carried out (Lucas et al., 2013).
Banana starch gelatinizes within a temperature range similar to cereal starch (Montoya et al., 2014). In this research, the gelatinization temperature was lower than the value reported for flour (68 °C) and banana starch (66,41 °C) (Lucas et al., 2013). Pozo (2019) recorded a temperature of 65 °C for green banana starch. The differences in the values obtained for the gelatinization temperature can be attributed to the genetic conditions of the fruit, the climatic conditions of the crop, and the weeks of harvest (Montoya et al., 2014).
The quality of the starch subsequently affects the properties of the bioplastic. Water resistance is an important property for biodegradable films, especially when used as a protective barrier for food, where water activity and the possibility of film breakage are high (Moro et al., 2017).
The water absorption, solubility, and swelling power indices serve as indicators of the functional properties of starch (Rodríguez-Sandoval et al., 2012). The WAI, WSI, and SP values were 4,73 + 0,19 g gel/g sample, 3,12 + 0,56%, and 4,81 + 0,18% respectively. Solubility and swelling power depend on the fruit's state of ripeness. High starch content and high viscosity entail low solubility, high water absorption, and high swelling power (Aristizábal and Sánchez, 2007).
The amylose and amylopectin contents for the banana peel flour were 8,59 + 0,82% and 91,41 + 0,82%, respectively. Pelissari et al., (2012) registered 23,10 and 35% for the amount of amylose in banana flour and starch. In the same way, Contreras-Pérez et al. (2018) obtained values between 23,5 and 31,3% for banana starch from four different varieties. The contents of amylose and amylopectin in starch are determining factors for the quality of finished foods. High values in amylose content favor greater solubility and a greater tendency to retrograde gels (Aristizábal and Sánchez, 2007). Amylose retrogrades faster than amylopectin because, due to its linear and highly polar nature, it tends to form hydrogen bonds between hydroxyl groups of adjacent molecules, thus causing a partial shrinkage of the starch (Salinas-Moreno et al., 2003). Therefore, the higher the content of amylopectin, the lower the retrogradation and, therefore, the better the properties of the bioplastic material formed.
The characterization of the extracted cellulose pulp is in Table 3.
The results obtained for cellulose were compared with those indicated for a wheat straw with 57,09% (Romero-Uscanga et al., 2014), which was used as a reinforcement material in thermoplastic materials, providing mechanical properties similar to those of composites of wood flour (Mishra and Sain, 2009). Similar experiences show satisfactory results when using other agro-industrial residues such as cocoa pod husk (Lubis et al., 2018) and rice straw (Bilo et al., 2018) as sustainable fillers in bioplastics.
With thermoplastic mixtures, the film was formed at basic pH (8,11-13,15) but not at neutral pH values (7,3). The increase in the concentration of the NaOH solution favored the consistency and durability of the material. Díaz et al. (2019) characterized chickpea flour films and found that film-forming solutions at pH 10 had better results than solutions at neutral pH.
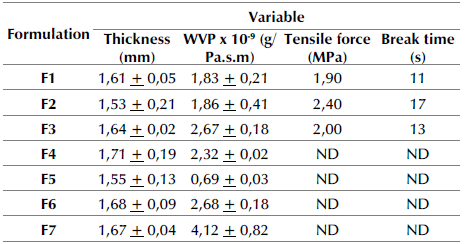
In the mechanical test, only formulations 1, 2, and 3 (F1-3) showed no breakage. In the rest of the formulations, the tensile strength and the breaking time could not be determined (ND). Therefore, to evaluate the incidence of the filler material on the characteristics of the bioplastic, 0,50 g of cellulose were added to F1 and F2 (F6 and F7, respectively), as they were the ones that exhibited the best appearance.
The results of the thickness, WVP, tensile strength and, break time tests for each formulation are shown in Table 4.
The thickness of the biodegradable films was between 1,55 and 1,71 mm, showing different results regardless of the type of plasticizer used. In a study involving edible films, a thickness of 0,11 mm was reported when using 0,5% banana peel and a 0,17 mm thickness with 1,5% banana peel (Anchundia et al., 2016). The permeability to water vapor showed values between 0,69 and 4,12x10-9 g/Pa.sm. However, in all cases, they were higher than 2,41x10-11 g/Pa.sm, which was recorded for a film made with a banana skin and salicylic acid (Anchundia et al., 2016). The addition of cellulose favors the passage of water vapor and, according to Wang et al. (2018), this occurs because cellulosic materials absorb or desorb the humidity from the surrounding air until they reach an equilibrium moisture content.
According to the results, both plasticizers reduce the passage of water vapor, but sorbitol does so to a lesser extent when it is not mixed with glycerol. F2 showed the best mechanical properties, with a tensile strength of 2,40 MPa and a break time of 17 seconds. The mechanical behavior of the material obtained in this research was better than that of bioplastics made with cassava starch, fique fiber, and glycerol, whose tensile stress was less than 2 MPa (Navia-Porras and Bejarano-Arana, 2014). On the other hand, it is lower than that reported for banana films, bark, and acetylsalicylic acid, whose tensile strength was between 4,43 and 10,80 MPa (Anchundia et al., 2016).
The influence of the plasticizer on the properties of the bioplastic was verified with an analysis of variance (ANOVA). The Shapiro-Wilk test was used to verify the normality of the data. For WVP and thickness, p-values of 0,105 and 0,9185 were obtained, respectively, in compliance with the normality assumption. The Bartlett test was also applied to verify the homoscedasticity of the variances. For thickness and WVP, p-values of 0,2787 and 0,4593 were obtained, respectively, satisfying the assumption of the equality of variances. In both tests, both the tensile strength and the breaking time had a p-value<0,05. For the tensile strength and the breaking time, given the characteristics of the data, the Kruskal-Wallis test was conducted.
The above-mentioned procedure was repeated to determine the influence exerted by cellulose on the characteristics of the material obtained. Fisher's test was used for the thickness and WVP variables. The corresponding results for both factors (plasticizer used and addition of cellulose) are shown in Table 5.
The ANOVA and the non-parametric test carried out for the plasticizer factor indicate, with a p-value<0,05, that there are significant differences in water vapor permeability, tensile strength, and break time, thus confirming the influence exerted by the type of plasticizer on the behavior of these variables. In similar studies, it was concluded that the concentration of the plasticizer affects the WVP and that it is positively correlated with the concentration of glycerol (Díaz et al., 2019; Faradilla et al., 2018). In other related research works, it was determined that plasticizers influence the physical properties of soy-based bioplastics (Tummala et al., 2006) since they increase the tensile modulus and tensile strength when using sorbitol instead of glycerol and when using a mixture of these intermediate values, in comparison with the results obtained when the plasticizers are used independently. For the cellulose addition factor, there was a p-value<0,05 in the WVP, indicating that the variable is affected when cellulose is added to the bioplastic film, as it was experimentally verified. On the contrary, the thickness did not show significant results, that is, it is not affected by the type of plasticizer used or by the addition of cellulose.
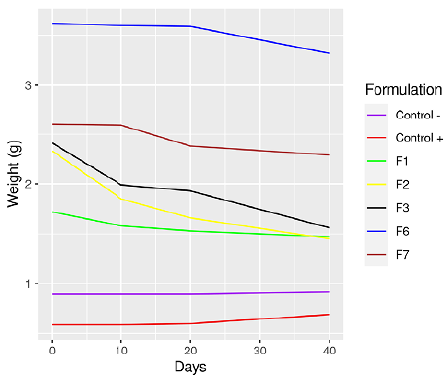
In the biodegradability test, information was only obtained for five of the formulations. At the end of the test, the samples were fragmented, thus making them difficult to review. To compare the behavior of the bioplastic produced against other materials, a positive control (filter paper) was taken as well as a negative control (PET plastic bottle). The weight loss during the 40 days of the biodegradability test are presented in Figure 1. Likewise, the total weight loss percentage of the bioplastics at the end of the test is shown in Table 6.
A decrease in the weight of the bioplastics was observed throughout testing, being more evident during the first ten days. The best result was for F2 with a weight loss of 37,77%, and the lowest value corresponded to F6 with 8,28%. The negative value in both the positive and negative controls implies a weight gain, probably due to the presence of moisture or traces of dirt. In a similar study, a weight loss of 64,21% was reported after 90 days in compost for a bioplastic made with potato starch (Meza et al., 2016). On the other hand, in the evaluation of a bioplastic based on a banana peel for agricultural purposes, 65,10% of the initial weight was lost in a period of eight weeks; its degradation process is much faster compared to other commercial plastics intended for sowing (Huzaisham and Marsi, 2020).
Conclusions
Bioplastics made from plant or animal biomass represent an alternative to replace synthetic plastics. In this research, banana residues were used to obtain thermoplastic starch. To extract starch and cellulose from the residues, physical, and chemical methods were applied. According to the results obtained during experimentation, it was concluded that starch has characteristics similar to those reported in previous works. The plasticizer used affected the WVP and the mechanical properties of the material. Although cellulose is used as a filler material to add strength, in this case, it increased the WVP, an unwanted characteristic in packaging materials. The biodegradability test showed that the material degrades for the evaluated conditions. The formulation with the lowest WVP was obtained when using glycerol, which works as a reference in food packaging applications. Regarding the mechanical properties, the best results were obtained when mixing glycerol and sorbitol. Although the developed formulations do not conclusively constitute a replacement for currently existing synthetic plastics, the results of this research serve as a reference for future work, where other formulations are evaluated, aimed at obtaining bioplastics capable of competing with synthetic plastics.