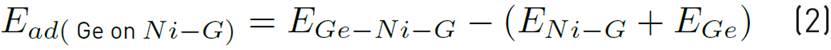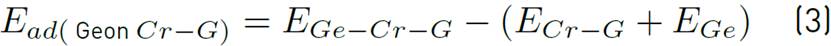1. Introduction
Genistein is a flavone compound. The highest amount of flavonoids has been found in soybean; for example, soy cheese or soy drinks[1-4], and it is obtained by fermentation or digestion of soybean[5]. It can be used for injunction multiplication of cancer cells. It is also applied to prevent heart diseases[6-8]. There are many analytical methods such as US-visible spectroscopy, mass spectroscopy, liquid chromatography, and biocompatible superparamagnetic drug delivery system, nano-formulation system, and nanoparticles for the determination of genistein[9-11]. Drug delivery systems prosper the pharmacokinetic and pharmacodynamics properties of different types of drug molecules[12]. Moreover, nanoparticles can develop the therapeutic potential of some established anticancer drugs. It is noteworthy that the benefit of using nano techniques in anticancer therapy is the objective delivery of anticancer drugs with few side effects[13-15].
Notably, nano techniques can be employed to receive genistein to the specific cancer cell[16-17] and, graphene has unique physical, mechanical, electrical and chemical properties[18-21]. Accordingly, the use of graphene as an adsorbent for biological molecules has been studied. For example, Ning Ding et al. deliberated the adsorption of nitrated tyrosine on the exclusive graphene and Nanometal- decorated graphene using two configurations of phenolic ring and gnitro group coordination on the graphene and found that when Au-Cr and Ni-doped graphene chemisorption occurred, the phenolic ring tended to the physisorption on the pristine graphene[22]. In another study, Zhang and et al. investigated the adsorption of H2 on the Ti-Zn-Al and N-doped graphene sheet, and they concluded that Ti had the largest amount of interaction energy (near -0.299 eV) and Zn-doped graphene sheet (approximately -0.294 eV) and Al-doped graphene sheet (about -0.13 eV). N-doped graphene did not have an effect on adsorption energy to improve adsorption H2 molecule on graphene sheet[23]. Shadmani et al. scrutinized the adsorption of MCM-41 on the graphene sheet by B3LYP/6-31G* and M05-2X/6-31G* basis set at different distances and studied the HOMO and LUMO energies, properties structure, ChelpG have been performed by DFT methods[24], and Shokuhi Rad examined the adsorption of Methanol and ethanol on the surface of t-decorated graphene by (DFT) calculation and discovered higher adsorption energies, low connecting distances, and high orbital hybridizing over adsorption of MeOH and EtOH molecules on PtG surface, the significant shift created in the location of the HOMO and LUMO[25], Shokuhi Rad also mulled over the (DFT) calculation to absorb some boron compounds (B(OH3))3, BF3 and (BCL3) on the surface of pristine and N-doped graphene , and found that N-doped graphene has higher adsorption energy and net charge transfer amount than pristine graphene. In another study, Shokuhi Rad and et al. dissected the adsorption of (NO2) and (N2O) molecules on the exclusive graphene and Al-decorated graphene by using (DFT) calculations.
The results presented that Al-doped graphene has substantial adsorption energy in compression of pristine graphene for the sake of existing the orbital hybridization between N2O as well as N2O, and Al-doped graphene, the adsorption energy for a most permanent state of NO2 and N2O was -62.2 kJ/mole and -33.9 kJ/mole[26-28]. Monajjemi considered liquid-phase -exfoliation (LPE) of graphite towards graphene theoretically and they indicated that sulfonic groups in a surfactant are most effective for any dissipation in the LPE process. They found that aromatic and ionic surfactant interacts with different carbon material, and p-stacking fall within their electron-abundant aromatic cores and conjugated surfaces of the carbon materials[29-31]. graphene material has attractive pure multi-physics, electrical[32], mechanical[33], and adsorption properties[34]. The graphene material is also responsible for modifying the charge carrier density and controlling its carrier mobility[35]. Since adsorption of genistein has not been studied on the surface of Ni-Ti-Cr-Se decorated graphene sheet, the present research aims at investigating and comparing the total adsorption energy and Gap energy for decoration of metallic atoms on the surface of graphene sheet in order to specify which element can be effected on the total and gap energy of adsorption. Egap or Gap energy is an energy range in a solid where no electron states can exist, especially in condensed-matter physics; an energy gap is often known more abstractly as a spectral gap, a term which needs not be specific to electrons or solids. If an energy gap exists in the band structure of a material, it is called a band gap.
The physical properties of semiconductors are determined by their band gaps to a large extent, but also for insulators and metals, and the band structure. Thus, any possible band gaps govern their electronic properties. Graphene, being a gapless semiconductor, cannot be used in pristine form for Nano-electronic applications. Therefore, it is essential to generate a finite gap in the energy dispersion at Dirac point. We present here the metal-binding model considering various interactions for tuning band gap in graphene. Full-field measurements can also be used to retrieve heterogeneous properties by minimizing the equilibrium gap.
2. Computational methods
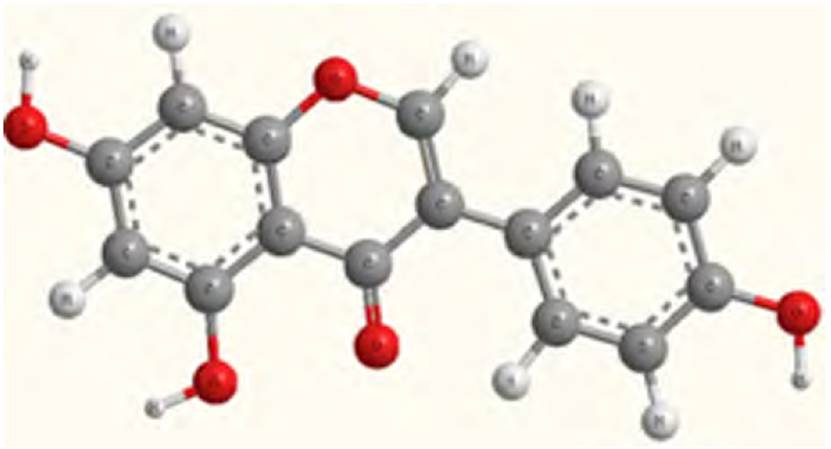
Genistein (4’,5,7-trihydroxy isoflavone) is a molecule with isoflavone groups obtained from soybean extraction [Figure 1]. In this study, we used 5 kinds of basis set for calculating total adsorption energy; pristine graphene used PM3MM and MINDO basis set, and for Cr, Se decorated graphene sheet used PM3 basis set. ZINDO basis set was utilized to measure total adsorption energy for Ti, Ni-decorated graphene sheet. First, we considered a (4×4 graphene supercell) [Figure 2 & Figure3) and then placed the genistein molecule in parallel with the graphene sheet by using HYPER CHEM software and converted it to ChemBio3D format to change distances between genistein and graphene decorated sheet. By dint of Equation (1), we obtained the total adsorption energy of genistein on pristine graphene:
Where:
EGe-G: Total electronic energies of interacting graphene with genistein
EGe: Total energy of pristine genistein.
EG: Total energy of pristine graphene.
For decorated graphene, we placed 9 atoms of Ni, Ti, Cr, and Se Individually on the graphene sheet [Figure 3] and obtained the total adsorption of genistein on Ni, Ti, Cr, and Se decorated graphene by using Equation (2), (3), (4), (5):
Where EGe-Ni-G, EGe-Cr-G, EGe-Ti-G and EGe-Se-G are the total electronic energies of graphene, and Ni, Cr, Ti and Se decorated graphene interacting with the genistein.

Figure 3 a) Ni-decorated graphene, b) Ti-decorated graphene, c) Cr-decorated graphene, d) Se-decorated graphene
Furthermore, we also obtained EHOMO, ELUMO, and Gap energy for genistein adsorption on Ni, Ti, Cr, and Se decorated graphene. All calculations were performed by the Gaussian09 program.
3. Results and discussion
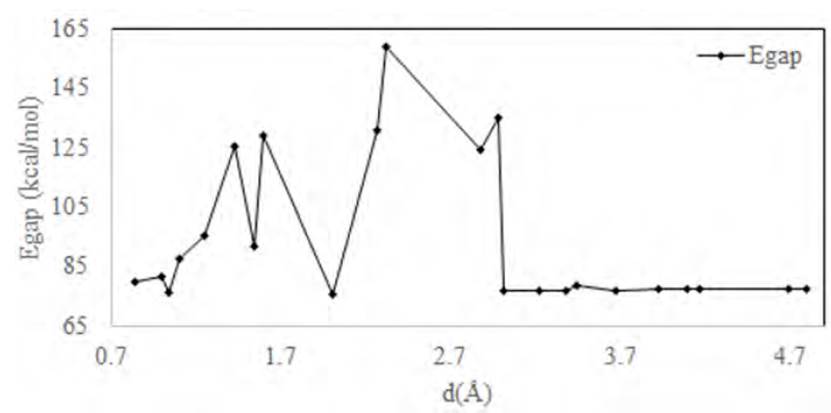
Primarily, we placed the genistein molecule on the surface of pristine graphene and then changed the distances of genistein from pristine graphene (0.8-5 Å). The calculated values of gap energy, EHOMO, and ELUMO, as well as the equilibrium distance of genistein graphene are listed in Table 1. As can be seen from the values of gap energy given in Table 1, upon adsorption of genistein on pristine graphene energy of EHOMO, ELUMO at distances between (0.8- 2.9 Å) decreased and increased after d=3.18 Å; they declined and became constant in 77 kcal/mole or 0.122 eV. (See Figure 3]. Table 1 displays the results of data and illustrates that the amount of minimum Egap for adsorption genistein on pristine graphene is 75.263 Kcal/mole, a favorable distance from genistein - graphene (de=2.265OA.). According to Figure 4, the maximum amount of Egap for adsorption genistein on pristine graphene is (159.148 kcal/mole or 0.25 eV). The calculated values of adsorption energy in the equilibrium distance (de=2.265OA.) of genistein to graphene is 954.984 Kcal/ mol.
3.1 Ni decoration on graphene sheet
We replaced nine atoms of carbon on the graphene sheet with Ni and placed the genistein molecule at different distances from the surface of Ni-decorated graphene d= (0.8-5Å) (see Figure 1]. We also measured EHOMO, ELUMO and Egap between distances (0.8-5 Å) genistein from Ni-decorated graphene (see Table 2]. The calculation demonstrated that the maximum amount of Egap for Ni-decorated graphene is 85.422 kcal/mole or 0.136 eV in the 1.178OA from genistein distance and Ni-decorated graphene, the minimum value of Egap at for Ni-decorated is 60.629 kcal/mole or 0.096 eV in the 3.918OA from genistein distance and Ni decorated graphene. Equation (6) is applied to make a connection between conductivity and Egap. As a result, Ni has higher electric conductivity and reactivity in comparison with C.
Where δ represents the electric conductivity and K is the Boltzmann constant[36]. The calculated values of the adsorption energy in the equilibrium distance (de=3.918OA.) of genistein to Ni decorated graphene is 318.168 Kcal/mol. Figure 5 shows that increasing distances Egap for adsorption genistein on Ni-decorated graphene in more than five distances reached about 64 kcal/mole, but between d=0.839-1.180Å.
Table 2 The energies of EHOMO, ELUMO, Egap-band for Ni-decorated graphene and its complexes with genistein at different distances

3.2 Ti decoration on graphene sheet
We superseded nine atoms of carbon on the graphene sheet with Ti and placed the genistein molecule at different distances from the surface of Ti-decorated graphene d= (0.8-5Å) (see Figure .1). The calculated values of gap energy, EHOMO, and ELUMO, as well as the equilibrium distance of genistein graphene listed in Table 3. The results obtained from the calculations of Table 3, as well as Figure 6, illustrate that Egap was in a minimum amount only at 4 specified distances (d=1.739, d=1.811, d=1.854 Å) and at d=3.478 Å, Egap was in a maximum amount about (92.476 kcal/mole or 0.147 eV) and for the other distances, it was stable, and no significant change was observed. Considering distances about (3.067 - 5.112 Å), the variation of Egap was from 59.832 -63.899 kcal/mole). The maximum amount of Egap for Ti decorated graphene is more than Ni and Cr maximum value of Egap and minimum value of Egap for Ti-decorated graphene about 2.4% fewer than Ni and Cr-decorated graphene exhibiting more conductivity and higher reactivity, in comparison with Ni and Cr decoration graphene. As shown in Figure 5, variations of Egap with changing distances is almost invariable and about 55-60 kcal/mole. Nonetheless, the minimum value of Egap gained just in d=1.854Å, (25.815 kcal/mol) and the maximum Egap gained at d=3.478. Indeed, it is the opposite of Ni-decorated graphene because Ni gained the maximum amount of Egap at d=1.081-1.178Å. The calculated values of adsorption energy in the equilibrium distance (de=1.854OA.) of genistein to Ti-decorated graphene is 797.480 Kcal/mol.
3.3 Cr decoration on graphene sheet
We replaced nine atoms of carbon on graphene sheet with Cr and placed the genistein molecule at different distances from the surface of Cr-decorated graphene d= (0.8-5Å) (see Figure 1]. When we used Cr for decoration graphene, it did not have a considerable change in total adsorption energy because the adsorption energy was higher than the other distances at d=0.886Å. Furthermore, we calculated the Egap, EHOMO and ELUMO listed in Table 4. As indicated in Table 4, the amount of EHOMO changed from -0.290 eV or -182.26 kcal/mole to -0.104 eV or -65.40 kcal/mole. Figure 7 presented that the maximal amount of Egap occurred at d=1.666Å, Egap=102.396 kcal/mole, and the minimum value of Egap=61.947 kcal/mole at d=3.669Å. Since they are less than the pristine graphene, the lower of Egap for Cr-decorated graphene corresponds to its higher conductivity and higher reactivity. The total adsorption energy was obtained at 946.725 kcal/mole.
3.4 Se decoration on graphene sheet
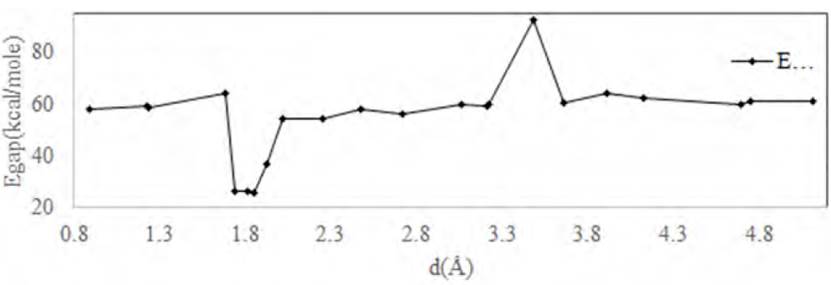
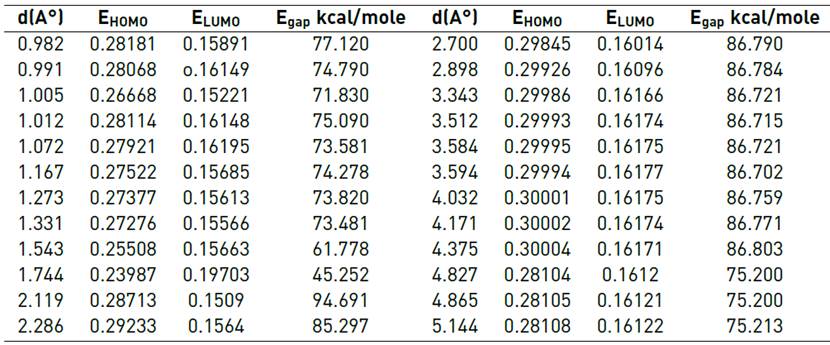
We displaced nine atoms of carbon on the graphene sheet with Se and placed the genistein molecule at different distances from the surface of Se-decorated graphene d= (0.8-5Å) (see Figure1). Table 5 features out the energy of EHOMO, ELUMO, and Egap for adsorption genistein on Se-decorated graphene.Figure 7 shows that the maximal amount of Egap occurred at d=2.119Å, Egap=94.691 kcal/mole, and the minimum value of Egap=45.252 kcal/mole at d=1.744Å. As they are less than the pristine graphene, the lower of Egap for Se-decorated graphene corresponds to its higher conductivity and higher reactivity. Moreover, Egap has little reduced at d=4.827 to 5.144 Å, and also, the maximum value of Egap for Se- decorated graphene is lower than that maximum value of Egap for pristine graphene; therefore, it is the same for a minimum amount of Egap. As a result, this reduction in the Egap implies more conductivity of decorated graphene by Se in compression with pristine graphene (see Figure 8]. Consequently, the lower Egap for Se-decorated graphene corresponds to its higher conductivity and higher reactivity. Its adsorption energy was obtained at 958.154 kcal/mole.
We can see that the maximum amount of Egap for Ni-decorated graphene is 85.42 kcal/mole or 0.136 eV. Thus, this amount is lower than Egap of Cr-decorated graphene and also Egap of pristine graphene. This trend proved that Ni has higher conductivity and reactivity in compression with C, the minimum value of Egap for Ni-decorated is 60.629 kcal/mole or 0.096 eV that is near the lowest amount of Egap for Cr-decorated graphene and also less than the minimum amount of Egap for pristine graphene. The calculated values of gap energy and adsorption energy, as well as the equilibrium distance of genistein to any atom (Ni, Cr, Ti, and Se) decorated graphene as listed in Table 6. This table displays that the arrangement of adsorption and gap energy for different atoms is achieved as: Ead genistein to Ni-decorated graphene ˂ Ead genistein to Ti-decorated graphene˂ Ead genistein to Cr-decorated graphene ˂ Ead genistein -PG ˂ Ead genistein to Se- decorated graphene. This order in the value of adsorption energy is in good agreement with the equilibrium of their distance of genistein surface.
Table 6 Values of gap energy, and Adsorption energy, as well as the equilibrium distance of genistein to any atom: (Ni, Cr, Ti, and Se) decorated graphene

Based on our calculations, the amounts of gap energies and also adsorption energies are listed in Table 6. Apparently, genistein- Ni-G complex, energetically, has a favorable position compared with other decorated graphenes. Therefore, the density of states (DOS) as well as frontier molecular orbitals HOMO and LUMO have been explained [Figure 9:a to Figure 9:f) to perfectly understand these structures such as Ni-decorated graphene - genistein, complex. The NBO analysis contributed to confirm the positive charges of Ni decorated graphene metal stability results. HOMO and LUMO energies of decorated Ni on graphene are listed in Table 6. As can be seen, HOMO and LUMO energies are increased to -0.23 and -0.13 at d=5.112 Å. and the gap band (EHOMO-ELUMO) decreased from 83.26 to 64.94 (kcal/mole).
As observed in Figure 9:a to Figure 9:f, the HOMO and LOMO gap energies of the system are obtained in Table 2 with swelling distance between genistein from Ni-decorated graphene. In comparison with other atoms, the electrical conductivity of the system becomes higher for Ni-decorated graphene by increasing Egap.
4. Conclusion
Using drug delivery methods in biotechnology, the adsorption of genistein on the surface of exclusive graphene and Ni, Ti, Cr, and Se doped graphene can bring about some significant information. Our calculations have been accomplished theoretically using density functional theory. We investigated the adsorption energy of the genistein molecule on the surface of pristine graphene and Ni, Cr, Ti and Se decorated graphene. The calculated values of gap energy and Adsorption energy, as well as the equilibrium distance of genistein to any atom (Ni, Cr, Ti, Se) decorated graphene, demonstrate that the order of adsorption and energy gap for different atoms is achieved as: Ead genistein to Ni-decorated graphene ˂ Ead genistein to Ti-decorated graphene˂ Ead genistein to Cr-decorated graphene ˂ Ead genistein -PG ˂ Ead genistein to Se decorated graphene. This order in the value of adsorption energy is in good agreement with the equilibrium of their distance of genistein surface. Subsequently, our results presented that the pristine graphene is the weakest adsorbent for genistein. Henceforth, we measured the maximum and minimum of Egap for genistein adsorption of Ni-Cr-Ti-Se decorated graphene sheet and the order of reactivity from higher to less is achieved as Ni>Ti>Se>Cr>pristine graphene. Apparently, the Ni atoms are more effective on reactivity and conductivity and can be a good choice for genistein adsorption on the graphene sheet towards the Cr-Ti and Se atoms.