Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.1 Bogotá Jan./Mar. 2015
Hepatoportal Sclerosis as a Cause of Portal Hypertension in a Colombian HIV patient without Cirrhosis
Luis Eduardo Barrera Herrera MD. (1), Mónica Tapias M. MD.(2), Víctor Idrovo C. MD. (2), Enrique Andrade R. MD. (3), Rocío del Pilar López R. MD (4)
(1) Laboratory and Pathology Department at the Hospital Universitario Fundación Santa Fe de Bogotá in Bogotá, Colombia.
(2) Gastroenterology, Hepatology and Transplant Services at the Hospital Universitario Fundación Santa Fe de Bogotá in Bogotá, Colombia.
(3) Laboratory and Pathology Department at the Hospital Universitario Fundación Santa Fe de Bogotá and at the Faculty of Medicine of the University of the Andes and the National University of Colombia in Bogotá, Colombia.
(4) Laboratory and Pathology Department at the Hospital Universitario Fundación Santa Fe de Bogotá and at the Faculty of Medicine of the University of the Andes in Bogotá, Colombia.
Case Report Poster Presentation: Hepatoportal Sclerosis as a Cause of Portal Hypertension in an HIV patient without Cirrhosis. R. López, Tapias M., Andrade R. XXXIX Colombian Congress of Pathology SOCOPAT, Colombian Society of Pathology, Santa Marta, Colombia 2012
Received: 26-06-14 Accepted: 02-02-15
Abstract
Background: Hepatoportal sclerosis manifests as non-cirrhotic portal hypertension. Its etiology appears to be related to alterations in the idiopathic micro-vasculature of the liver. Manifestations of hepatoportal sclerosis include upper gastrointestinal bleeding, pancytopenia, splenomegaly and non-cirrhotic portal hypertension. We present the first reported case of hepatoportal sclerosis in Colombia which occurred in an HIV positive patient.
Methods: A 60-year-old male HIV patient positive was admitted to our institution because of ascites and upper digestive tract bleeding due to esophageal and fundal varices. Management required taking a liver biopsy.
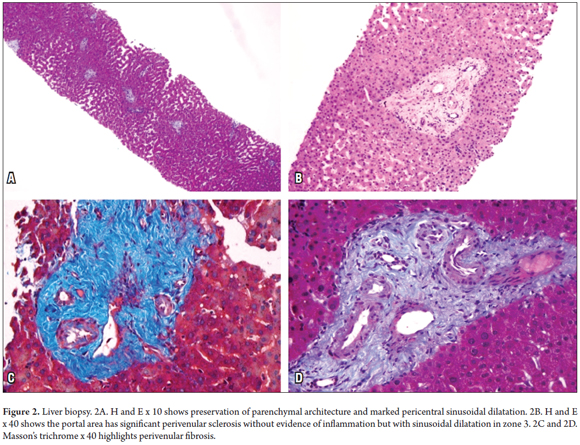
Results: A Tru-Cut biopsy needle was used to take a liver biopsy sample percutaneously. The biopsy revealed six to eight portal tracts with preserved architectural parenchyma, perivenular fibrosis and severe pericentral sinusoidal dilatation.
Conclusions: Hepatoportal sclerosis is a cause of morbidity in HIV-positive patients and should be considered in each patient manifesting non-cirrhotic portal hypertension associated with upper gastrointestinal bleeding. However, further research is necessary to describe the relationship between the development of intrahepatic alterations (microthrombosis), HIV, and the use of anti-retroviral therapy, particularly the use of didanosine.
Keywords
Tru-cut liver biopsy, hepatoportal sclerosis, non-cirrhotic portal hypertension, human immunodeficiency virus, anti-retroviral therapy.
INTRODUCTION
The main causes of morbidity and mortality in patients with liver disease whose blood tests positive for HIV are hepatitis B and hepatitis C. Alcoholic and nonalcoholic steatohepatitis and toxicity due to antiretroviral therapy are also frequently involved. Less commonly involved is non-cirrhotic portal hypertension (NCPH). The prolonged use of didanosine for antiretroviral therapy in patients whose blood tests positive for HIV is commonly associated with liver disease (1). In at least 100 cases reported in the literature, nodular regenerative hyperplasia and hepatoportal sclerosis (HPS) are the most common findings (2). Although the pattern of liver damage associated with antiretroviral therapy is not well established, there have been reports of patients with hepatoportal sclerosis who required liver transplantation (3, 4).
PATIENTS AND METHODS
A 60 year old male patient who had first tested positive for HIV 15 years earlier after an episode of disseminated histoplasmosis was admitted to the emergency department because of an episode of hematemesis secondary to bleeding esophageal varices and bleeding in the fundus associated with the presence of ascites. Paraclinical studies taken at admittance showed an aspartate aminotransferase (AST) level of 58 IU/L (5-34 IU/L), an alanine transaminase (ALT) level of 50 IU/ ml (<55 U/L), an alkaline phosphatase level of 164 IU/L (40-50), total bilirubin of 46 mg/dL (0.2-1mg/dL), direct bilirubin of 0.17mg/dL (<0.3 mg / dL), indirect bilirubin of 0.29 mg/dL, a platelet count of 168 (103)/mm3 (150-450 (103) /mm3), INR of 1.05, an HIV viral load, and a CD4 count of just 280 cells. The patient tested negative for both hepatitis B and hepatitis C. Antiretroviral medications being taken by the patient included zidovudine, didanosine and efavirenz which he had taken for over 14 years. In addition, the patient had been taking medicine for high blood pressure, gastritis, hypercholesterolemia, and spondylitis including metoprolol, aspirin, sulfasalazine, atorvastatin, clopidogrel, allopurinol and colchicine for 3 years.
Digestive tract endoscopy revealed linked esophageal varices. Administration of 40 mg/day of low molecular weight heparin (enoxaparin) was started. Doppler ultrasound (Figure 1A and 1B) showed that the liver had signs of chronic liver disease but did not have any focal lesions or signs of cirrhosis. Portal vein thrombosis extended from the splenic mesenteric venous confluence until the parenchymal portal vein branches. The permeability of the hepatic veins was. Portal hypertension was indicated by a perihepatic collection of fluid, splenomegaly and thickening of the vessel walls. A presumptive diagnosis of NCPH was made.
Analysis of ascitic fluids revealed an albumin gradient greater than 1.1 g/dl, but cultures were negative for common infections including by mycobacteria and fungi. Drugs that could potentially cause liver toxicity including atorvastatin and sulfasalazine were suspended. A liver biopsy was performed without complications (Figure 2). The patient experienced episodes of encephalopathy which were probably of hepatic origin secondary to non-cirrhotic portal hypertension and its clinical manifestation, Bradypsychia which alters the sleeping-waking cycle and memory. While hospitalized, the patient's symptoms improved, but one year later he suffered massive gastrointestinal hemorrhaging and died.
RESULTS
A series of slides were made from tissue obtained from trucut biopsies and stained with H & E, PAS-diastase, Masson's trichrome, aldehyde fuchsin, Kinyoun and reticulin and a test for iron.
Microscopic Study
The architecture of the hepatic parenchyma was intact, and eight portal spaces with marked perivenular portal fibrosis could be seen. Occasional lymphoid cells could be seen, but there was no evidence of necrosis of the hepatocytes or of chronic or acute cholestasis. The most relevant finding was pericentral sinusoidal dilation in zone 3 (Figures 2A and 2B). Hypertrophic Kupffer cells with brown pigment that were intensely reactive with PAS-diastase were also observed. A cytoplasmic liposfuscin pigment was identified in zone 3. It was positive according to its coloring with Kinyoun stain, and slightly positive with PAS-diastase and aldehyde fuchsin. Tests for iron and copper were negative. The Masson trichrome showed portal perivenular fibrosis (Figures 2C and 2D) with trabecular focal atrophy due to regenerative nodular hyperplasia.
DISCUSSION
NCPH is a rare condition with clinical manifestations of portal hypertension including esophageal varices, portal hypertensive gastropathy, ascites, and splenomegaly, but without cirrhosis. The cause of portosystemic shunt in these cases has not been clearly established, but hepatic encephalopathy may occur in the absence of cirrhosis. This in turn is manifested primarily by neuropsychiatric symptoms. Furthermore portal thrombosis can sometimes be the cause of portal hypertension leading to episodes of encephalopathy of varying intensity. The most widely accepted hypothesis for the mechanism involved is that astrocyte cell edema is produced by the metabolism of ammonia in the brain (5-8).
Among the entities that have been described as causes of NCPH are thrombosis of the portal vein, nodular regenerative hyperplasia, incomplete septal cirrhosis, different types of vasculitis and schistosomiasis (9-12). Although the etiology of NCPH has not been established, multifactorial mechanisms have been proposed to explain its pathogenesis. Alterations in the pre-hepatic portal vasculature due to endothelial cell damage caused by HIV and the use of drugs such as didanosine have been described (13). Prothrombotic states due HIV infections have also been described as the direct cause of protein S antibodies which give rise to protein S deficiency which results in obliteration of portal venules and regeneration and hepatic hyperplasia (14). Presinusoidal portal hypertension also appears as the hemodynamic profile of NCPH in which there is a normal or slightly elevated hepatic venous pressure gradient (<10 mmHg) (15).
Over the past 10 years we have found several reports of HIV positive patients with symptoms of NCPH, some of whom required liver transplantation. As mentioned, the relationship between HIV and NCPH seems to be multifactorial. Prothrombotic states and especially antiretroviral treatment using didanosine have been postulated as causes of this disease in HIV-positive patients. Didanosine is the most frequently associated factor in the literature (1, 4). Apparently cumulative dosage or idiosyncratic mechanisms (15) or even polymorphisms in genes involved in the metabolism of didanosine may predispose patients for hepatic veno-occlusive disease (16).
Little is known about the exact mechanism that causes damage to hepatocytes from antiretroviral treatment. Published cases show how idiopathic cirrhosis with portal hypertension begins with a damage to smaller vessels and progresses to changes in the architecture of the hepatic parenchyma with nodular regenerative hyperplasia (NRH) (1). While portal vein thrombosis is a common finding in patients with NCPH (17), it could be either a cause or an effect of hepatic decompensation (16). HPS has been described as the underlying mechanism for NCPH in HIV-positive patients. It has been attributed to intrahepatic microthrombosis or abnormalities of liver fibrogenesis due to antiretroviral treatment or as part of the damage caused by HIV (2). In our case, the histological findings describing portal fibrosis and sinusoidal dilatation were the only alterations found in the microscopic study which explained the obstruction of the venous flow and hence the associated symptoms.
Globally, HPS has proven to be a clear cause of morbidity in HIV-positive patients and, given its high potential for mortality, it must be considered in any patient who manifests NCPH with upper gastrointestinal bleeding (18). In the literature, most reported cases include patients with upper gastrointestinal bleeding whose liver function tests are normal and who also have been treated with didanosine for long periods of time (19). This is related to mitochondrial damage and consequent damage to hepatic vascular endothelial tissue (17).
CONCLUSION
Although some years ago liver diseases of unknown causes in HIV seropositive patients were unusual, today there are increasing numbers of reports of them with NCHPS being the most common finding. The case reported here is the first reported in Colombia of HPS in a patient with HIV. It underlines the importance of the microscopic interpretation and its correlation with clinical and radiological studies. In our case all of these together were necessary to explain the signs and symptoms of NCPH in our patient that corresponded to an HPS. Neuropsychiatric symptoms, non-cirrhotic portal hypertension and the possibility of a portosystemic encephalopathy should all be remembered in the differential diagnosis of these patients.
Finally, this report highlights the need to develop further research to establish the relationship between HIV, antiretroviral treatment and liver disorders. This is especially important because there is insufficient evidence to recommend anticoagulation therapy and supplementary assessment of prothrombotic states for these patients.
Conflicts of Interest
The authors declare that they have no conflicts of interest with regard to the publication of this article.
REFERENCES
1. Kovari H, Ledergerber B, Peter U, Flepp M, Jost J, Schmid P, et al. Association of noncirrhotic portal hypertension in HIV-infected persons and antiretroviral therapy with didanosine: a nested case-control study. Clin Infect Dis Off Publ Infect Dis Soc Am 2009;49(4):626-35. [ Links ]
2. Schiano TD, Kotler DP, Ferran E, Fiel MI. Hepatoportal sclerosis as a cause of noncirrhotic portal hypertension in patients with HIV. Am J Gastroenterol 2007;102(11):2536-40. [ Links ]
3. Isabel Fiel M, Thung SN, Hytiroglou P, Emre S, Schiano TD. Liver failure and need for liver transplantation in patients with advanced hepatoportal sclerosis. Am J Surg Pathol 2007;31(4):607-14. [ Links ]
4. Krasinskas AM, Eghtesad B, Kamath PS, Demetris AJ, Abraham SC. Liver transplantation for severe intrahepatic noncirrhotic portal hypertension. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc 2005;11(6):627-34; discussion 610-1. [ Links ]
5. Fukushima K, Kurozumi M, Kadoya M, Ikeda S. Portal-systemic encephalopathy in a non-cirrhotic patient. BMJ Case Rep 2009;2009:bcr2007121822. [ Links ]
6. Fukushima K, Kurozumi M, Kadoya M, Ikeda S. Neurological picture. Portal-systemic encephalopathy in a non-cirrhotic patient. J Neurol Neurosurg Psychiatry 2008;79(1):96. [ Links ]
7. Watanabe A. Portal-systemic encephalopathy in non-cirrhotic patients: classification of clinical types, diagnosis and treatment. J Gastroenterol Hepatol 2000;15(9):969-79. [ Links ]
8. Mínguez B, García-Pagán JC, Bosch J, Turnes J, Alonso J, Rovira A, et al. Noncirrhotic portal vein thrombosis exhibits neuropsychological and MR changes consistent with minimal hepatic encephalopathy. Hepatol Baltim Md 2006;43(4):707-14. [ Links ]
9. Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatol Baltim Md 2006;44(1):7-14. [ Links ]
10. Sciot R, Staessen D, Van Damme B, Van Steenbergen W, Fevery J, De Groote J, et al. Incomplete septal cirrhosis: histopathological aspects. Histopathology 1988;13(6):593-603. [ Links ]
11. Nayak NC, Ramalingaswami V. Obliterative portal venopathy of the liver. Associated with so-called idiopathic portal hypertension or tropical splenomegaly. Arch Pathol 1969;87(4):359-69. [ Links ]
12. Ludwig J, Hashimoto E, Obata H, Baldus WP. Idiopathic portal hypertension; a histopathological study of 26 Japanese cases. Histopathology. 1993;22(3):227-34. [ Links ]
13. Saifee S, Joelson D, Braude J, Shrestha R, Johnson M, Sellers M, et al. Noncirrhotic portal hypertension in patients with human immunodeficiency virus-1 infection. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2008;6(10):1167-9. [ Links ]
14. Alvarez Díaz H, Mariño Callejo A, García Rodríguez JF. Non-cirrhotic portal hypertension in human immunodeficiency virus-infected patients: a new challenge in antiretroviral therapy era. Open AIDS J 2011;5:59-61. [ Links ]
15. Chang P-EJ, Garcia-Pagan J-C. Idiopathic noncirrhotic portal hypertension in HIV-infected patients. Clin Infect Dis Off Publ Infect Dis Soc Am 2010;50(1):127-8; author reply 128-9. [ Links ]
16. Mendizabal M, Craviotto S, Chen T, Silva MO, Reddy KR. Noncirrhotic portal hypertension: another cause of liver disease in HIV patients. Ann Hepatol 2009;8(4):390-5. [ Links ]
17. Walker UA, Setzer B, Venhoff N. Increased long-term mitochondrial toxicity in combinations of nucleoside analogue reverse-transcriptase inhibitors. AIDS Lond Engl 2002;16(16):2165-73. [ Links ]
18. Castellares C, Barreiro P, Martín-Carbonero L, Labarga P, Vispo ME, Casado R, et al. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat 2008;15(3):165-72. [ Links ]
19. Vispo E, Moreno A, Maida I, Barreiro P, Cuevas A, Albertos S, et al. Noncirrhotic portal hypertension in HIV-infected patients: unique clinical and pathological findings. AIDS Lond Engl 2010;24(8):1171-6. [ Links ]











 text in
text in