Introduction
Helicobacter pylori is a helical gram-negative bacillus with a worldwide prevalence of more than 50% in low- and middle-income countries and less than 50% in high-income countries1. In Colombia, the frequency varies according to different studies (41.7%-99.1%)2-4. H. pylori is associated with inflammatory and neoplastic gastroduodenal diseases and has been recognized as a definitive gastric carcinogen since 1994, according to the IARC (International Agency for Research on Cancer)5. In 2020 in Colombia, gastric cancer (GC) ranked second in incidence in men (4,989) and fifth in women (3,225)6. Chronic H. pylori infection leads to atrophic gastritis and intestinal metaplasia (IM), both considered preneoplastic lesions (PNL) of GC, which are potentially reversible when early and effective treatment is instituted to eradicate the bacterium7,8. Therefore, early diagnosis and therapeutic management of the infection can reduce the risk of GC.
For H. pylori diagnosis, there are non-invasive and invasive methods. The former indirectly detect the bacterium or its products and include serology (sensitivity [Sen] = 76%-84% and specificity [Spe] = 79%-90%), fecal antigens (Sen = 69%-95% and Spe = 97.6%)9, and the urease breath test (Sen = 96%-100% and Spe = 93%-100%)10. The latter are based on upper GI endoscopy (EGD) with biopsies and include the rapid urease test (Sen = 80%-95% and Spe = 95%-100%), microbiological culture (Sen = 70% and Spe = 100%), molecular tests (Sen = 91% and Spe = 100%), and histopathology (Sen = 90%-95% and Spe = 95%-98%)11. Histopathology has several advantages because it allows the diagnosis of the infection, determines the degree of inflammation of the gastric mucosa, and evaluates the presence of PNL12.
On the one hand, for the histopathological study of H. pylori, it is advisable to carry out standardized sampling (Sydney protocol) in which several biopsies are taken from specific sites of the stomach, given the heterogeneous distribution of the bacteria that can lead to false negatives when selecting a single sampling site11. On the other hand, the stain routinely used for histopathological diagnosis is hematoxylin and eosin (H&E). This stain is usually sufficient in high-density infections, although it has a variable sensitivity between 69% and 93%; however, the density of H. pylori decreases with increasing PNL13, so in patients with PNL, the sensitivity of H&E is less than 70%14. It has been reported that the exclusive use of H&E bypasses H. pylori with low density15.
The implementation of the Sydney protocol improves the sensitivity and specificity in the diagnosis of H. pylori16. It consists of evaluating five samples, two from the antrum, two from the gastric body, and one from the angular incisure, which increases the probability of finding the bacterium. A previous study published by our group determined the presence of H. pylori not only in the gastric antrum but also in other samples used in the Sydney protocol17.
Assessment of intestinal atrophy and metaplasia are best determined in the region of the angular incisure, which is also the site most likely to reveal dysplastic changes; hence the importance of this biopsy. This protocol, added to the use of special stains such as Giemsa, Alcian Blue, Periodic Acid Schiff (PAS), or Warthin-Starry, improves diagnosis, especially in patients with PNL1,14,18.
Chahuan et al. demonstrated that, besides H&E, Giemsa staining is preferred because it is sensitive (42.6%-94%), easy to perform, cheap, widely available, and does not produce precipitates that can be confused with the bacterium15. Several studies show the superiority of Giemsa staining compared to H&E, whose sensitivity ranges from 28.7% to 83.9% in detecting the bacterium15,18,19. In addition, Giemsa staining reduces interobserver variability in the diagnosis of infection because it facilitates its viewing17,20.
Up to 75% of atrophic gastritis cases are associated with H. pylori. Still, the detection of the bacterium can go unnoticed21, causing false negative results in the diagnosis and not allowing the patient to receive timely treatment for eradication22, as demonstrated in previous studies22-26.
In Colombia, no studies were found on determining the usefulness of special stains such as Giemsa for diagnosing H. pylori in patients with gastric PNL. Therefore, this study aimed to assess the effectiveness of Giemsa staining for diagnosing H. pylori in gastric biopsies of patients with PNL who attended seven healthcare institutions in three regions of Antioquia, Colombia, during 2016-2018.
Materials and methods
Study population and eligibility criteria
This study derives from the CODI 2014-1062 project approved by the Research Ethics Committee of the Medicine School, Universidad de Antioquia. Individuals ≥18 years of age who attended seven healthcare institutions in three subregions of Antioquia (Valle de Aburrá metropolitan area, Oriente, and Urabá Antioqueño) were included. The participants came for EGD performance, and participation in the project was voluntary by signing the consent form.
We excluded individuals who received proton pump inhibitors (PPIs) or H2-histamine receptor antagonists during the 15 days before EGD or antibiotics within the last month; individuals diagnosed with upper gastrointestinal bleeding; anticoagulated patients or patients with coagulation disorders; pregnant women; people with previous surgical history in the upper GI tract; prior diagnosis of chronic severe diseases (renal, hepatic, decompensated heart failure, and decompensated diabetes mellitus), and people with a history of radiochemotherapy. From 272 individuals included in the previous study, a sample of 65 patients with a histopathological diagnosis of PNL (atrophic gastritis or intestinal metaplasia) was selected for this study. Patients with dysplasia were not included since this histopathological finding was not reported in the patients included17.
Biopsy collection and processing
Five samples were taken from each participant following the recommendations of the updated Sydney protocol that include a sample of the greater curvature of the antrum (A1), lesser curvature of the antrum (A2), angular incisure (I), greater curvature of the body (C1), and lesser curvature of the body (C2); a sixth sample was taken in cases where a tumor was found. Samples were stored and transported to the Las Vegas Clinic Cytology and Pathology Unit for processing. Samples from each patient were stained with H&E and modified Giemsa-Diff Quick.
Biopsy reading and histopathological diagnosis of H. pylori
All five Giemsa-stained biopsies were evaluated and blinded-read randomly by one of two pathologists and a third-year pathology resident. The presence or absence of the bacterium was determined as positive or negative by directly observing helical gram-negative bacilli with a light microscope (Leica® DM500). For its quantification, the visual analog scale of the updated Sydney protocol was used (absent, scarce, moderate, and abundant)16. The bacteria was searched in non-atrophic areas without intestinal mucosal metaplasia. The staining characteristics of the bacterium to specify in the H&E staining were monochromatism similar to the foveolar epithelium and, in the special Giemsa staining, the dark blue stain that stands out. In cases of discrepancies in the bacterium presence or quantification, the second pathologist and the pathology resident completed a third reading, agreeing upon the results.
Data analysis
The statistical package SPSS (IBM) v. 27 was employed. For the qualitative variables, we used the absolute and relative frequency distribution of the categories of the variables. The mean ± standard deviation (SD) was used for the quantitative age variable since a normal distribution was observed according to the Kolmogorov-Smirnov test. The ratio between the two stainings was defined based on a 2 x 2 table dividing the number of positive and negative matches by the total.
Results
The study evaluated 325 gastric biopsies from 65 patients with PNL. The mean age was 54.4 years (SD: 16.4), and 63% (41) were female; 69.2% (45) of the participants lived in the metropolitan area of Medellín, 23.1% (15) in Oriente, and 7.7% (5) in Urabá Antioqueño.
Regarding the histopathological diagnosis of H. pylori infection, Giemsa staining had a positivity rate of 98.5%, lower than H&E (Figure 1).
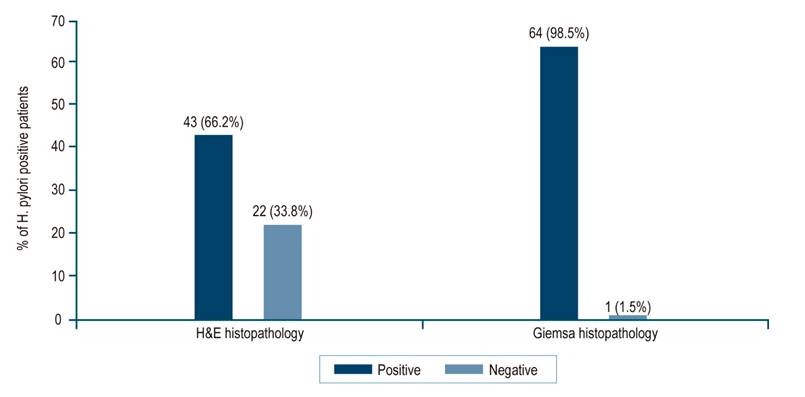
Figure 1 Distribution of patients according to the results of histopathological staining for H. pylori. Source: The authors.
When analyzing the presence of the bacterium at the biopsy site, H. pylori was more frequent in A1, A2, and I. The positivity for the diagnosis of H. pylori was higher with Giemsa staining in all anatomical sites (61.5%-72%), while H&E varied between 41.5% and 49% (Figure 2).

Figure 2 Distribution of positive H&E and Giemsa results for H. pylori by anatomical site. A1: greater curvature of the antrum; A2: lesser curvature of the antrum; I: angular incisure; C1: greater curvature of the body; C2: lesser curvature of the body. Source: The authors.
The proportion for positive and negative results between H&E staining and Giemsa staining was higher in the C1 samples, with 86.1%, and lower in A1, with 73.8% (Figure 3).
The proportion of samples positive for H. pylori evaluated with Giemsa staining was higher than H&E. The difference was more significant in the samples with a low amount of bacteria, with a statistically significant difference (p = 0.035) (Table 1).
Table 1 The proportion of positivity for H. pylori according to the infection density and staining used in each anatomical site
| Sampling site | Amount of H. pylori | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scarce | Moderate | Abundant | ||||||||||
| H&E | Giemsa | H&E | Giemsa | H&E | Giemsa | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| A1 | 13 | 8.5 | 23 | 10.5 | 11 | 7.2 | 12 | 5.5 | 8 | 5.2 | 12 | 5.5 |
| A2 | 8 | 5.2 | 21 | 9.6 | 13 | 8.5 | 10 | 4.6 | 9 | 5.9 | 12 | 5.5 |
| I | 10 | 6.5 | 25 | 11.4 | 10 | 6.5 | 12 | 5.5 | 10 | 6.5 | 9 | 4.1 |
| C1 | 15 | 9.8 | 18 | 8.2 | 9 | 5.9 | 13 | 5.9 | 9 | 5.9 | 11 | 5.0 |
| C2 | 14 | 9.1 | 19 | 8.7 | 8 | 5.2 | 13 | 5.9 | 6 | 3.9 | 9 | 4.1 |
| Total | 60 | 39.1 | 106 | 48.4 | 51 | 33.3 | 60 | 27.4 | 42 | 27.4 | 53 | 24.2 |
A1: greater curvature of the antrum; A2: lesser curvature of the antrum; I: angular incisure; C1: greater curvature of the body; C2: lesser curvature of the body. Number of positive cases for H&E: 153, and for Giemsa: 219. Source: The authors.
Discussion
Several invasive and non-invasive methods are available for detecting H. pylori. Still, no test is the gold standard for diagnosis if sensitivity, specificity, cost, reproducibility, and speed are considered11. This study used two histological techniques (H&E and Giemsa) to diagnose H. pylori in patients with PNL (atrophic gastritis and intestinal metaplasia), whose bacterial density is low. According to the results, the Giemsa stain had a higher proportion of positivity in each of the five anatomical sites evaluated according to the updated Sydney protocol. This proportion was consistently higher in the group of samples with scarce bacteria.
The use of complementary stains has been evaluated in previous studies. Khan H et al. reported the need for other stainings such as Giemsa, PAS-AB, Warthin-Starry, and immunohistochemistry (IHC) in patients with low bacterial load (mild inflammation) associated with atrophic mucosa or after eradication therapy27. In our experience, the Giemsa stain was selected as complementary since it is reported in the literature for detecting H. pylori given its characteristics: economical, sensitive, easy to perform, and reproducible9. In a 2014 study, Boldt et al. found that Giemsa staining had higher sensitivity and specificity when compared to H&E28. Alkhamiss AS et al. found that the specificity of H&E for H. pylori is high (91.18%). Still, its sensitivity is low (66.67%) compared to Giemsa staining, whose sensitivity and specificity was high (93.33% and 100%, respectively), which suggests that Giemsa is a better option when compared to H&E29.
This study noted that the H&E stain had a lower positivity rate (66.2%) compared to the complementary Giemsa stain (98.5%), as reported by Alkhamiss AS et al.29. For their part, Mawlood et al. said similar findings for Giemsa with a positivity rate of 93.5% and H&E of 83.9%17. Laine et al. found a similar sensitivity between the two stains (H&E: 92% and Giemsa: 88%). They highlighted that the specificity of Giemsa was significantly higher than the H&E stain (98% and 89%, respectively), which is why they recommend it for the diagnosis of H. pylori18.
The proportion of samples positive for H. pylori with Giemsa was consistently higher than that with H&E in samples with low amounts of bacteria (p = 0.035), with diagnoses of an average of 20.3% more positive cases. It suggests that the H&E stain should be supplemented with an additional stain, such as Giemsa, when the amount of bacteria is low, as demonstrated by Moayyedi et al.30 and by Vaira D et al. They determined that histological examination can miss low-density infections, mainly if performed only with H&E31. In these circumstances, the bacterium can easily be confused with cellular debris since H&E staining is not specific for H. pylori. Sabbagh P et al. proved that the accuracy of the histopathological diagnosis of H. pylori depends on the number and location of the biopsies collected11.
This study could diagnose the H. pylori infection in samples from the five anatomical sites, which is consistent with what was reported by Lee JY et al. They mention that in cases of atrophic gastritis and intestinal metaplasia, there is a change in the usual colonization of the antrum towards the proximal stomach (body of the antrum and gastric fundus) as a result of hostile antral conditions, including increased pH, in which atrophy and metaplasia occur more frequently. The authors also reported that the gastric body is the appropriate biopsy site to detect H. pylori in patients with these lesions32.
In Colombia, there is no consensus on the histological diagnosis of H. pylori. Sabbagh P et al. report that this method could make the diagnosis with a single gastric biopsy sample. However, multiple biopsies are recommended to increase diagnostic accuracy and sensitivity11. Generally, two different staining methods are employed: H&E for evaluating inflammatory cells and Giemsa for viewing the bacteria11. Alkhamiss AS et al., Makristathis et al., and Batts KP et al. suggested that studies complementary to H&E, such as Giemsa staining for the diagnosis of H. pylori, should be performed only if the presence of an infection by the bacterium that cannot be viewed with H&E is highly suspected, such as cases with active gastritis or the formation of germinal centers29,33,34.
Lee JY et al. also report that the specificity of histology can be improved by special stains such as Giemsa and immunohistochemical stains32, the latter being used in other countries in cases of low bacterial density, atrophic gastritis with extensive intestinal metaplasia and chronic active gastritis without identification of H. pylori by standard staining. The IHC is more specific; however, it is more expensive, more technically challenging, and unavailable in all laboratories9.
According to the Colombian Association of Gastroenterology clinical practice guideline for diagnosing and treating H. pylori in adults, routine basic staining with H&E and special staining with Giemsa is recommended to determine the presence or absence of H. pylori. IHC is reserved for cases with negative staining, active inflammation, post-treatment biopsies of MALT lymphomas, and when coccoid forms or other organisms cannot be identified with certainty35. According to Kocsmár É et al., the use of IHC is reasonable in cases that are negative with Giemsa staining and do not exhibit inflammatory activity and in which the etiological role of H. pylori is suggested by clinical, anamnestic, or other data36.
Conclusion
In low- and middle-income countries, such as Colombia, it is increasingly critical that health systems find cost-effective and efficient alternatives to diagnose H. pylori. Giemsa staining proved helpful in the histopathological study of H. pylori in samples with low bacterial density, such as those from patients with PNL; however, its usefulness in evaluating non-atrophic gastric mucosa in ulcers and neoplasias is not ruled out. Giemsa staining could increase the sensitivity of the infection diagnosis and, thus, optimize the bacterium eradication treatment to reduce or reverse its progression to disease.











 text in
text in 




