Introduction
The liver plays a critical role in protein synthesis and the storage of glycogen and fat-soluble vitamins, in addition to breaking down xenobiotic compounds, such as drugs.1 Certain commonly used medications and substances, including over-the-counter drugs, have been known to cause toxic hepatitis.2
Liver disease caused by substances and drugs can mask the symptoms of acute and chronic liver diseases,3-6 with varying degrees of severity. Although it is a rarely reported condition, toxic hepatitis has high morbidity and mortality rates and presents a challenge in terms of diagnosis and timely treatment, as it is usually detected only after liver damage has occurred.3,4 Drug-induced liver injury is one of the most severe adverse reactions and is the leading cause of hepatotoxicity in many locations.5
When attributing hepatoxicity to supplements and drugs, it is necessary to exclude other causes, such as viruses, bacteria, autoimmunity, metabolic and vascular diseases, alcohol, biliary pathology, and neoplasms, before making the diagnosis.4,6 Although hepatoxicity induced by natural products is not commonly reported, it has piqued the interest of clinicians,2,3 making it essential to document new cases. For example, some nonsteroidal anti-inflammatory drugs (NSAIDs) were withdrawn from the market due to severe hepatoxicity. These drugs accounted for almost 10% of cases of drug-induced liver disease, with seven NSAIDs being responsible for this adverse reaction.7
It is important to note that nimesulide, compared to other NSAIDs, causes a greater proportion and severity of adverse liver events.8 The risk of hepatoxicity due to nimesulide also increases with longer consumption time and higher doses than conventional.8,9
Based on the above, we are presenting a case report of a woman who developed toxic hepatitis associated with the consumption of an herbal supplement (Herbalife®) and concomitant use of nimesulide. The patient was treated at a high-level complexity institution in Pasto, Colombia.
Clinical case
The patient was a 51-year-old female and professional with a BMI of 25.64 who sought medical attention (internal medicine) for a mandibular nodule. She had no relevant medical record. The physician initially prescribed nimesulide at a dose of two tablets of 100 mg per day for two days, assuming it to be an inflammatory process. The patient reported nausea and jaundice on the third day while denying other symptoms such as abdominal pain, fever, diarrhea, or vomiting. During her admission to the emergency room, the patient disclosed a history of chronic Herbalife® supplement consumption for four years, with a daily intake pattern of shakes. The patient sought medical care at a third-level healthcare institution due to the worsening of symptoms. In the initial differential diagnosis, inflammatory and viral pathologies were considered.
The patient was hospitalized and underwent a comprehensive diagnostic workup to determine the cause of her symptoms. Laboratory tests revealed elevated levels of transaminases, coagulation times, and other liver markers, as shown in Table 1. The blood count did not show leukocytosis, and the lipase test could not be processed due to reagent-related issues. The physical examination revealed adenomegaly in the mandibular region while ruling out the presence of other skin lesions or acute bleeding. The toxicology and internal medicine services evaluated the case, and a diagnosis of jaundiced syndrome with possible hepatoxicity was made.
Table 1 Complementary serum tests performed on the patient
| Laboratory parameter | Admission | Day 7 | Day 10 | Discharge |
|---|---|---|---|---|
| Alkaline phosphatase | 341 U/L | 298 U/L | 258 U/L | 86 U/L |
| Amylase~ | 22.5 U/L | No data | No data | No data |
| Total cholesterol | 175 mg/dL | No data | No data | 172 mg/dL |
| Triglycerides | 167 mg/dL | No data | No data | 180 mg/dL |
| GGT | 109 U/L | 95 U/L | No data | 60 U/L |
| Total bilirubin | 10.8 mg/dL | 2.90 mg/dL | No data | 2.0 mg/dL |
| Direct bilirubin | 6.7 mg/dL | 0.00 mg/dL | No data | 1.2 mg/dL |
| Indirect bilirubin | 4.1 mg/dL | 1.10 mg/dL | No data | 0.8 mg/dL |
| Glycemia | 88 mg/dL | 110 mg/dL | No data | No data |
| Creatinine | 0.69 mg/dL | No data | No data | No data |
| ALT | 1160 U/L | 987 U/L | 574 U/L | 272 U/L |
| AST | 786 U/L | 780 U/L | 513 U/L | 180 U/L |
| PT | 15.8 s | 16.7 s | 13 s | No data |
| PTT | 65 s | 31 s | 31 s | No data |
| Leukocytes | 8555 10³/μL | No data | No data | 6700 10³/μL |
| Hb | 14.6 g/dL | No data | No data | 12 g/dL |
| Hto | 43.4% | No data | No data | 40.2% |
| Platelets | 130 10³/μL | No data | No data | 150 10³/μL |
| *Hepatitis B and C negative Embryonal carcinoma antigen CA 19 negative Negative anti-smooth muscle Ab | HIV - negative serology | Negative CMV IgG- IgM | Negative Epstein-Barr IgG-IgM | Negative antinuclear, mitochondrial, and microsomal Ab |
~There was no amylase follow-up due to the lack of reagent in the institution. *The clinical history reported that tests for hepatitis B and C (in addition to the last row) were non-reactive; however, numerical parameters were not provided as the patient’s insurance company had the tests performed at an external institution, and the institutional report was qualitative. Ab: antibodies; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CA: cancer; CMV: cytomegalovirus; GGT: gamma-glutamyl transferase; Hb: hemoglobin; Hto: hematocrit; IgG: immunoglobulin G; IgM: immunoglobulin M; PT: prothrombin time; PTT: partial thromboplastin time; HIV: human immunodeficiency virus. Source: HUDN (Hospital Universitario Departamental de Nariño) Clinical Laboratory.
In this case, the most relevant differential diagnoses considered were autoimmune hepatitis, pesticide-derived lesions (which were ruled out based on the patient’s history and symptoms), hepatotropic viruses, alcoholic hepatitis, and biliary pathology.
The case study started with a neck ultrasound to assess the mandibular nodule, which incidentally revealed a subcentric right thyroid nodule and rounded adenomegaly in the right submandibular region with loss of a suspicious cortex-marrow relationship. Tests were then performed for hepatotropic viruses and other conditions, which yielded negative results. A fine-needle aspiration biopsy (FNAB) of the submandibular nodule showed indeterminate atypia of lymphoid cells. Based on these findings, the patient was treated with vitamin K (10 mg intravenously/day), hydration (Ringer’s lactate), and antibiotics (ampicillin/sulbactam 3 g intravenously every six hours).
After the liver and bile duct ultrasound, hepatic steatosis, cholecystitis, and biliary sludge were detected. Consequently, the surgeons assessed the patient and requested both abdominal tomography (Figure 1) and magnetic resonance cholangiopancreatography (Figure 2). However, as hyperbilirubinemia persisted at the expense of direct hyperbilirubinemia, gastroenterologists performed an endoscopic retrograde cholangiopancreatography (ERCP). The ERCP revealed the papilla of Vater in the second flat duodenal portion, selective admission to the bile duct with bow papillotome plus hydrophilic guide. In addition, the contrast-enhanced cholangiography confirmed a normal caliber bile duct without stones, exploration of the bile duct with Dormia basket and extraction of biliary sludge and retained bile, and bile duct washing until clear bile drainage.
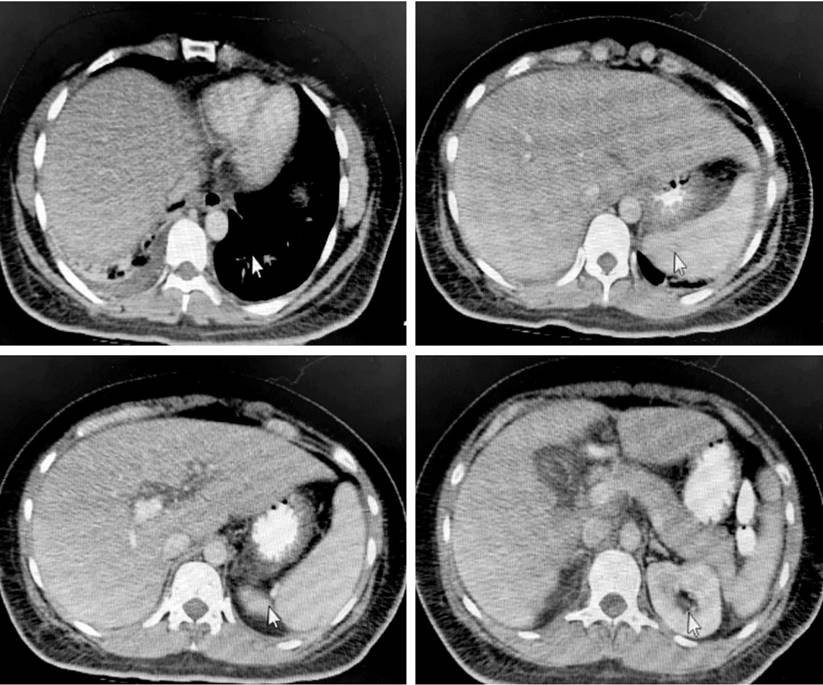
Figure 1 Computed tomography (CT) scan of the patient’s abdomen. Right laminar pleural effusion with subsegmental atelectasis. Diffuse hepatic steatosis without focal lesions. Findings described at the pancreatic level suggest interstitial edematous process Balthazar C. Severity index 2. Right renal hypodense nodular image compatible with a cyst. Intra-abdominal laminar fluid. Courtesy of HUDN Radiology Department
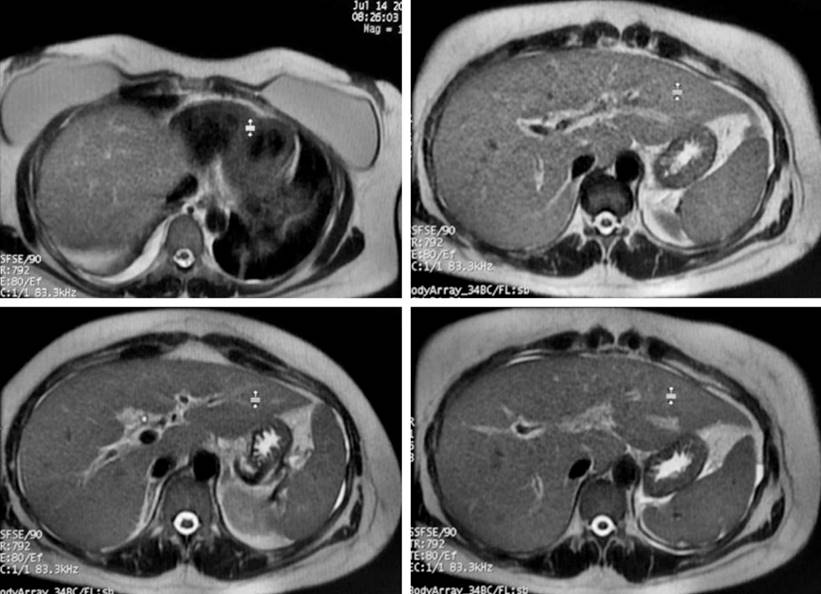
Figure 2 Magnetic resonance cholangiopancreatography of the patient. Edema of the gallbladder walls and biliary sludge inside. Stones that may cause obstruction are not observed in the vesicular lumen or the bile duct. Free liquid in Morrison’s space and right parietocolic leak. Free fluid in the right lung base. Mild hepatomegaly. Courtesy of HUDN Radiology Department.
The patient had a non-obstructive liver condition with no stones in the bile duct. She improved due to her asymptomatic state, stable hemodynamics, decreased values of paraclinical controls (specifically bilirubin and transaminases), absence of an evident infectious process, and resolution of the mandibular adenopathy.
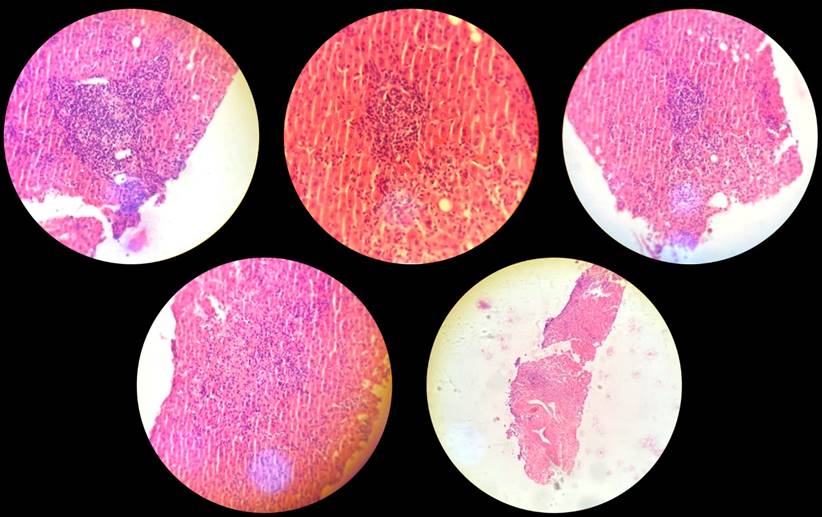
The patient underwent a liver biopsy (Figure 3), and the histopathological diagnosis revealed an inflammatory process of white line cells, which was compatible with a pattern of acute hepatitis with pericentral necrosis and apoptotic hepatocytes. It is important to highlight that the patient’s clinical condition improved after discontinuing nimesulide medication and the dietary supplement. Thirty days after discharge, follow-up tests showed that liver function and transaminases were within normal parameters. In conjunction with the patient’s clinical evolution, it indicates that the medication was the cause of the pathological process.
Discussion
Toxic hepatitis can have various causes, such as the consumption of medications or xenobiotics like immunosuppressants, anti-tuberculosis drugs, antibiotics, and nonsteroidal anti-inflammatory drugs (NSAIDs).10 Analgesics are responsible for most cases of drug-induced liver injury.10 In fact, up to 15% of patients using NSAIDs may experience at least one episode of transient elevation of transaminases.11 Risk factors associated with the development of drug-induced liver injury include advanced age (60 to 70 years), female sex, pre-existing liver disease, and genetic polymorphisms in drug metabolism pathways according to ethnicity.5,8
The use of dietary and herbal supplements has become increasingly popular and has consequently had an impact on liver damage.12 For instance, the consumption of Herbalife® products has been associated with varying degrees of liver damage, ranging from minimal changes to fulminant hepatitis that requires transplantation.13 Herbal and dietary supplements contain a wide variety of ingredients, including vitamins, minerals, proteins, herbs, and other botanicals.13,14 The nutritional information of these compounds typically includes a mixture of minerals such as calcium citrate, magnesium oxide, ferrous fumarate, sodium selenite, zinc oxide, manganese carbonate, chromium chloride [III], potassium iodide, cupric citrate, and potassium phosphate. Additionally, anti-caking agents such as silicon dioxide and botanical products such as shiitake mushroom, green tea leaf, oolong tea leaf, black tea leaf, pomegranate peel, parsley, dandelion, milk thistle extract, Echinacea purpurea, Echinacea angustifolia, Astragalus, and Schisandra are also commonly found in these supplements.15
Although they appear natural and safe, it is important to note that the safety of these substances remains controversial, as there is limited evidence available on their efficacy and toxicity.14 Hepatoxicity resulting from the use of herbal products is often attributed to an idiosyncratic reaction rather than toxic damage.13
Recent publications have reported cases of toxicity caused by herbal supplements in various regions, including Asia (where traditional Chinese medicine is used), America (including the United States and Latin America), and Europe (such as Spain, Iceland, and France).14,16 Specifically, Herbalife® products have been found to contain agents suspected of causing hepatoxicity, such as green tea extract, ginkgo, and saw palmetto.12 In addition, hepatocellular damage is the predominant mechanism of liver damage caused by these extracts, followed by the mixed mechanism, among others.12,13,16 The severity of liver damage caused by herbal supplements varies from mild to severe, and in some cases, can even lead to cirrhosis and liver failure that requires liver transplantation.13
Dietary and herbal supplements can lead to metabolic activation via the liver cytochrome P450 complex or intestinal bacteria, resulting in the production of toxic metabolites that can bind to reduced cellular glutathione, forming potentially toxic protein/DNA adducts.17
According to a study, patients with acute liver failure caused by herbal and dietary supplements are more likely to require liver transplantation compared to those caused by medication prescription.18,19 Analyzing the toxicity of Herbalife® products can be complex as they may contain non-herbal chemical components that need to be evaluated as hepatoxicity precursors.20 For example, contamination with Bacillus subtilis in Herbalife® products could potentially contribute to the hepatoxicity profile of this herbal supplement.18
In comparison to other NSAIDs, nimesulide is known to have a higher incidence of hepatoxicity, according to studies.21,22 In fact, an integrative study found that approximately 45% of patients with adverse hepatic reactions due to nimesulide required liver transplantation or died from fulminant liver failure.21 The toxicity caused by nimesulide may have a latency period ranging from 90 days to six months.8 Recent studies have shown that most patients with nimesulide-induced hepatoxicity are women, elderly, and have jaundice,8,21 which is consistent with the case presented in this report.
The mechanism responsible for nimesulide-induced liver damage remains unknown, and genetic association studies have yet to be conducted.8) Potential mechanisms of hepatoxicity include the involvement of the adaptive immune system, bioactivation via oxidative stress production by nitroreductases, and the idiosyncratic metabolic hypothesis.8
However, further studies are needed to evaluate the risk of nimesulide-induced liver damage based on certain characteristics such as age, sex, dosage, and duration of treatment.21 It should also be noted that the concomitant use of certain drugs and xenobiotics can potentiate the hepatotoxic effect. For instance, like Herbalife®, nimesulide is metabolized by cytochrome P450 isoenzymes.17,22
The liver biopsy on the patient revealed the presence of mononuclear inflammatory infiltrate and areas of necrosis in the liver tissue, leading to the histopathological diagnosis of acute drug-induced hepatitis based on the clinical context. However, a limitation of the study is that the findings could not be expanded upon to accurately support the diagnosis from the pathology. The elevated levels of both transaminases and an ALT/AST ratio of 1.4 to 1.5 suggest that the hepatic condition could have mixed with cholestatic predominance, according to López-Gil et al.23
The analysis of this case suggests a potential synergistic mechanism of hepatoxicity with the concurrent use of nimesulide and the Herbalife® dietary supplement, given that other possible causes of hepatoxicity were ruled out. Currently, there are no studies evaluating the possibility of a joint mechanism of liver toxicity, as observed in this case. Additionally, it is important to emphasize the need for regulations in the prescription of hepatotoxic drugs and the use of products without long-term medical indications, which may synergistically affect organs involved in the metabolism and excretion of xenobiotics.











 text in
text in