Introduction
Gastroparesis is a chronic motility disorder characterized by poor gastric accommodation, leading to symptoms such as nausea, vomiting, early satiety, abdominal pain, and weight loss. As a sine qua non condition, it is essential to rule out mechanical obstruction and confirm delayed gastric emptying1. The annual cost associated with gastroparesis is approximately $568 million dollars2. In the United States, the prevalence is 21.5 cases per 100,000 inhabitants, with a higher incidence in females (female-male ratio 3.8:1) and in Caucasians (71.7%), followed by African Americans (15.2%), Hispanics (11.2%), and Asians (1.9%).
Diabetes mellitus is the primary cause (57.4%), followed by gastrointestinal surgical procedures, particularly those involving vagotomy (15.0%), medications (11.8%), idiopathic causes (11.3%), and miscellaneous causes (4.5%), which include connective tissue diseases3. The pathophysiology involves altered gastric motility or increased pyloric pressure. Disruptions in the accommodation of the gastric body and fundus, antral hypomotility, lack of pyloric relaxation, and small intestine dysmotility can result in delayed gastric emptying. Immune-mediated mechanisms likely play a critical role in the pathogenesis. Structural studies have reported lesions in the interstitial cells of Cajal (ICCs), nerves, and gastric smooth muscle cells, as well as the loss of macrophages with an anti-inflammatory phenotype4.
Diagnosis is made through solid-phase gastric emptying scintigraphy, which evaluates gastric emptying by ingesting food labeled with a radiotracer (technetium-99m [99mTc]). Interpretation is based on measuring the percentage and time of emptying (usually over 4 hours) and the percentage of retention of the labeled food. In patients with > 60% retention at 2 hours or > 10% at 4 hours, the study is considered conclusive for gastroparesis5.
Complementary methods include the breath test, where a substrate (octanoic acid or Spirulina platensis) labeled with a stable carbon isotope (13C) is added to a solid food portion. After digestion and absorption in the small intestine, these substrates are metabolized by the liver, exhaled as 13C through the lungs, and quantified by mass spectrometry4.
FDA-approved wireless motility capsules are sensitive for detecting delays in gastric emptying, though their clinical relevance is still debated. They also allow for the evaluation of abnormal intestinal transit and are valuable for addressing comorbidities such as constipation associated with gastroparesis4. Other techniques like magnetic resonance imaging and ultrasound are useful in research; however, their clinical use faces limitations due to factors like cost and equipment availability6.
Recently, the functional lumen imaging probe (EndoFLIP) has been validated as an attractive tool for measuring parameters in the pylorus, such as pyloric hypertonia, which is one of the potential pathophysiological mechanisms implicated in delayed gastric emptying. Its potential role in this field is being increasingly studied, given its usefulness in identifying the subtype of patients with pyloric dysfunction who could benefit from pylorus-directed therapies (Figure 1).
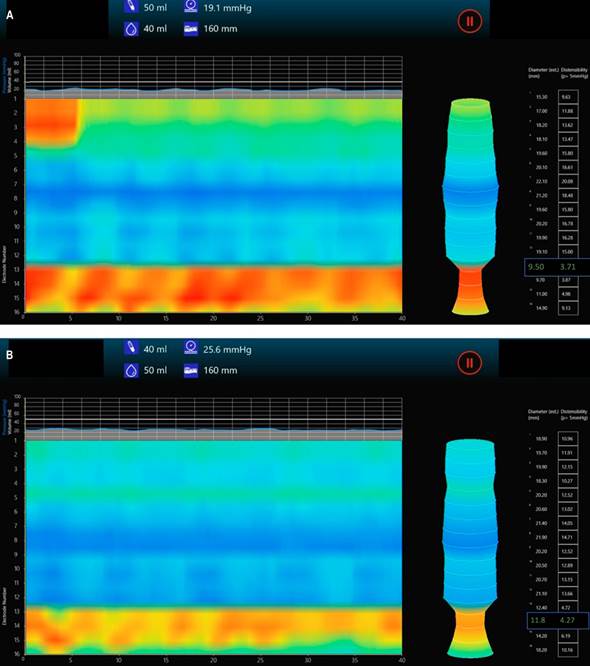
Author’s file.
Figure 1 Pyloric EndoFLIP with decreased diameter and pyloric distensibility index in a patient with gastroparesis. Images show (A) balloon inflated to 40 mL and (B) to 50 mL.
The treatment of gastroparesis involves a combination of pharmacological and non-pharmacological strategies. A fractionated diet (4 to 5 low-fat and low-fiber portions daily) is fundamental7, followed by the use of prokinetics such as metoclopramide, domperidone, and levosulpiride.
Despite these measures, up to 40% of patients develop refractory gastroparesis8, which the American Gastroenterological Association (AGA) defines as persistent symptoms despite dietary adjustments and at least four weeks of effective therapy with first-line prokinetics at an optimal dose and with adequate adherence9.
Alternative therapies have been employed, such as hydraulic balloon pyloric dilation (HBPD), botulinum toxin (BT), laparoscopic pyloromyotomy, and gastric pacemakers, with varying success rates (43% to 77%10, 83% to 86%11, and 53.7%, respectively12).
Gastric peroral endoscopic myotomy (G-POEM) has emerged as an innovative option with technical and clinical success and a positive impact on quality of life13. Despite an adverse event rate of 5%-10%14, G-POEM shows efficacy similar to laparoscopic pyloroplasty but with shorter procedure times and fewer complications, making it a potentially preferred choice for refractory gastroparesis, especially in those documented with pyloric hypertonia15.
The pyloric sphincter: a zone of special interest in gastric emptying
The pyloric sphincter is a narrow area of smooth muscle with increased luminal pressure located between the stomach and the duodenum. Its function is regulated by intrinsic innervation from the gastric myenteric plexus and extrinsic innervation through branches of the vagus nerve. Hence, pyloric dysfunction represents a key mechanism in gastroparesis, whose nature can vary depending on the underlying cause4. The contractions and relaxations of the pylorus depend on the transmission of nerve signals through the ICCs and the response of smooth muscle cells. There are at least two independently controlled functional areas: the circular muscle near the myenteric plexus, dominated by the propagation of gastric slow waves, resulting in sphincter contractions at the end of each peristaltic movement, and the deeper circular muscle, regulated by motor neurons, where slow waves have no influence. The pylorus has garnered increasing interest because its relaxation in synchrony with motor activity between the antrum and the duodenum facilitates gastric emptying8.
Endoflip device and its operational characteristics
EndoFLIP is a widely used diagnostic tool in the esophagus for evaluating the lower esophageal sphincter (LES). It utilizes a catheter with 16 sensors that, when inflated, measures diameter (D), pressure (P), cross-sectional area (CSA), and distensibility index (DI). The DI indicates the sphincter’s ability to stretch and is calculated by measuring the CSA at the narrowest point of the sphincter and dividing it by its P. There are two catheter models: EF-325 and EF-322, which differ in length (8 cm and 16 cm respectively) and the distance between their sensors (1 cm and 2 cm respectively). LES measurements have been standardized and are represented through the FLIP 1.0 and FLIP 2.0 modules as a 3D topographic image of the esophagus16.
Its use at the pyloric level shares similar procedural characteristics. After a minimum fast of six hours for non-clear liquids and two hours for water, the patient should be positioned in the left lateral decubitus position and sedated. A therapeutic endoscope is inserted through the esophagus into the stomach and antrum, where an endoscopic examination is performed before crossing the pylorus. Next, the EndoFLIP catheter (ideally the pyloric model EF-325N) is zero-calibrated and lubricated with conductive gel before being inserted through the working channel of the endoscope. Once the catheter is positioned in the pylorus under direct visualization, the balloon is inflated at a rate of 1 mL/s until reaching 40 mL-50 mL (optimal observation of the pyloric contour is obtained at 40 mL). Pressure is then measured for five seconds in mmHg using the pressure transducer located in the probe. For CSA measurement, conductive liquid is perfused through the infusion port at the proximal end of the probe, obtaining an impedance value by planimetry based on Ohm’s law (voltage = current x resistance); the diameter is then derived as the square root of 4 CSA/π. The DI (a measure indicating the ease of structure stretching) is derived from the ratio between the CSA and P at the narrowest point of the sphincter. Elasticity, providing information on the ease of stretching along the entire sphincter, is calculated similarly to the DI but considers the total volume from 1 cm above to 1 cm below the narrowest point of the sphincter. After completing the pyloric measurement, the catheter is withdrawn through the channel, and the upper endoscopic examination is completed with direct visualization of the pylorus and duodenum to rule out lesions caused by previous treatments and the examination itself17-19. It should be noted that general anesthesia may cause a slight increase in P and a decrease in pyloric DI, which should be corroborated with prospective trials20.
Pylorus measurements
For the accurate interpretation of measurements in the pylorus, it is crucial to consider two aspects. The first aspect is the position of the FLIP catheter balloon in relation to the pylorus. Three positions have been predefined: proximal (with 75% of the balloon in the duodenum and 25% in the antrum), mid (50% in the duodenum and 50% in the antrum), and distal (25% in the duodenum and 75% in the antrum). Yim and colleagues demonstrated that the balloon’s position relative to the pylorus results in different measurements of DI, CSA, and P (Table 1). Specifically, the measurement of P was significantly higher when most of the balloon was distal to the pylorus and folded in the second part of the duodenum compared to when most of the balloon was in the antrum. The pyloric DI is significantly lower when the balloon is primarily positioned in the duodenum compared to the stomach. These findings may be due to balloon deformation and changes in geometry or spatial arrangement when placed within the duodenum.
Table 1 Variation of EndoFLIP Measurements Related to the Balloon Position with Respect to the Pylorus
| Measurement | Inflation Volume (mL) | Proximal Position | Mid Position | Distal Position |
|---|---|---|---|---|
| CSA (mm²) | 40 | 130.1 | 137.9 | 115.0 |
| 50 | 183.0 | 190.2 | 185.8 | |
| Average pressure (mmHg) | 40 | 15.2 | 11.6 | 10.6 |
| 50 | 28.1 | 31.5 | 21.1 | |
| Distensibility index (mm²/mm Hg) | 40 | 9.8 | 13.9 | 13.1 |
| 50 | 6.7 | 9.4 | 9.6 |
Adapted from: Yim B and colleagues; J Neurogastroenterol Motil. 2023;29(2):192-99.
The second aspect concerns the geometry of the duodenal bulb. The FLIP catheter runs straight at the level of the LES. However, in the case of the pylorus, where esophageal EndoFLIP catheters are commonly used, the curved geometry of the duodenal bulb causes balloon deformation. Consequently, the sensors do not remain centered, affecting the CSA estimates of the pyloric sphincter through impedance planimetry. Since DI measurements are calculated from CSA, it is not surprising to see alterations in this parameter with different positions19. Therefore, it is concluded that positioning the FLIP catheter balloon primarily in the antrum (distal) minimizes deformation, allowing for more reliable measurements.
Several studies have addressed defining reference values for pyloric EndoFLIP measurements. Gourcerol and colleagues evaluated a cohort of healthy volunteers and found that the average DI was 25.2 ± 2.4 mm²/mmHg when inflating the EndoFLIP balloon to 40 mL, with no significant variations when measured at 10, 20, and 30 mL consecutively. This study also evaluated a subgroup of patients with gastroparesis of various etiologies (diabetic, idiopathic, and post-fundoplication) and found that the pyloric DI decreased significantly at all evaluated volumes, establishing a normality threshold value greater than 10 mm²/mmHg.
Zhen and colleagues evaluated another larger cohort of healthy volunteers and found D values of 13.0 ± 2.5, 14.3 ± 1.8, and 17.2 ± 2.0 mm, DI values of 10.9 ± 4.8, 11.3 ± 5.8, and 11.1 ± 4.3 mm2/mmHg, and P values of 12.5 ± 3.9, 15.8 ± 4.8, and 21.7 ± 5.3 mmHg for distensions of 40, 50, and 60 mL, respectively22.
Various series have evaluated these measurements in patients with gastroparesis. Jacques and colleagues23 and Malik and colleagues24 evaluated patients with diabetic gastroparesis (DG) and idiopathic gastroparesis (IG) and found DI values at 40 mL inflation of 11.7 and 10.0 ± 1.3 mm²/mmHg, respectively. Although the results obtained in these studies did not define a definitive cutoff point for DI with a high discriminative capacity between healthy and sick subjects, it appears that values below 10 mm²/mmHg could identify most patients with gastroparesis. The population with values between 10 and 25.2 ± 2.4 mm²/mmHg should be characterized in prospective studies, as those with values close to the lower limit could correspond to false negatives.
Desprez and colleagues compared pyloric DI among three groups: healthy volunteers, patients with DGP, and patients with IG. They also examined the clinical characteristics of the diabetic patients in detail. For this study, DI values greater than 10 mm²/mmHg were considered normal. The results showed that the DI measured with 40 mL inflation was lower in the DGP and IG groups, specifically 10.8 ± 0.9 mm²/mmHg and 14.8 ± 2.2 mm²/mmHg, respectively, compared to the healthy volunteer group, which had a DI of 25.2 ± 2.3 mm²/mmHg (p < 0.005). A reduced pyloric DI was observed in 56.5% of the DGP patients, 51.5% of the IG patients, and 10% of the healthy volunteers. No correlation was found between the pyloric sphincter measurements and diabetes-related characteristics, including blood glucose levels, glycated hemoglobin, type of diabetes mellitus, neuropathy, or the use of glucagon-like peptide-1 (GLP-1) agonists. Both DI and P of the pyloric sphincter were altered in DGP and IG, while distensibility did not correlate with diabetes parameters25. Table 2 summarizes the different variables measured in these studies.
Table 2 Pyloric EndoFLIP Measurements in Healthy and Gastroparesis Patients
| Study | Etiology of Gastroparesis | Inflation Volume (mL) | Average Pyloric Diameter (mm) | Distensibility Index (mm²/mm Hg) | Average Pressure (mmHg) | CSA (mm²) |
|---|---|---|---|---|---|---|
| Gourcerol, et al. | Healthy patients | 40 | - | 25.2 | - | - |
| Zheng, et al. | Healthy patients | 40 | 13.0 ± 2.5 | 10.9 ± 3.8 | 12.5 ± 3.9 | 138.6 ± 55.4 |
| 50 | 14.3 ± 1.8 | 11.3 ± 5.8 | 15.8 ± 4.8 | 162.4 ± 39.2 | ||
| Jacques, et al. | 50% diabetic, 20% idiopathic, 25% miscellaneous*, 5% post-surgical | 40 | 13.9 | 11.7 | 11.7 | 152.2 |
| 50 | 17.3 | 8.1 | 28.9 | 234.5 | ||
| Malik, et al. | 72% idiopathic, 28% diabetic | 40 | 12.2 ± 0.44 | 10.0 ± 1.3 | 18 ± 1.23 | 125.2 ± 9.15 |
| 50 | 14.1 ± 0.41 | 6.0 ± 1.3 | 32.5 ± 1.55 | 164.7 ± 10.2 |
*15% secondary to Sjögren’s syndrome, 5% to Parkinson’s disease, and 5% to systemic sclerosis. Author’s own research.
Based on these findings, subsequent research has focused on determining whether DI measurement could predict the clinical outcomes of therapies specifically targeting the pylorus, such as HBPD, BT, or G-POEM. For HBPD, it has been shown that patients who experience greater symptomatic relief had lower DI values before dilation (7.2 ± 1.0 mm²/mmHg)26. The EsoFLIP (dilation balloon with incorporated EndoFLIP) could be a useful strategy to improve the success rate of this procedure, as it safely allows surpassing the dilation target threshold of 18-20 mm; in selected cases, it achieves residual diameters of up to 30 mm without increasing the complication rate27.
Regarding the use of BT, DI values less than 8-10 mm²/mmHg predict a good symptomatic response post-intervention28. Saadi and colleagues also demonstrated the utility of EndoFLIP in predicting symptom response. Besides conventional measurements, they evaluated elasticity (very similar to DI, see the Operational Characteristics section). Patients with greater pre-BT elasticity showed marked improvement in nausea (r = -0.34, p = 0.03) and early satiety (r = -0.34, p = 0.04) at eight weeks. Interestingly, patients with higher preoperative DI experienced less abdominal pain eight weeks postoperatively, while those with lower DI, CSA, and D had more severe symptoms of nausea and vomiting18.
Recent studies have focused on evaluating the role of EndoFLIP in the treatment of patients who are candidates for G-POEM. Preoperative data from Jacques and colleagues23 showed that DI values below 9.2 mm²/mmHg predict clinical success at three months. Postoperatively, Vosoughi and colleagues demonstrated that CSA measurement appears to be the best predictor of clinical success and increased gastric emptying, with values greater than 154 mm² at a distension volume of 40 mL predicting clinical success at one year with a sensitivity and specificity of 71% and 91%, respectively20. Additionally, increases greater than 20% in CSA compared to the preoperative value correlate with clinical success30. This suggests that EndoFLIP could be used both before and after the procedure to help predict or confirm the efficacy of pyloromyotomy, guiding the procedure similarly to its use for the LES in patients with achalasia.
In Colombia, the limited number of specialized centers in gastrointestinal physiology and the high cost of EndoFLIP technology compared to other diagnostic strategies restrict its reproducibility and negatively impact the characterization and appropriate selection of pylorus-targeted therapies. Overcoming these obstacles could lead to its routine use and inclusion in local guidelines for managing patients with refractory gastroparesis.
Conclusion
Pyloric EndoFLIP is an attractive complementary diagnostic tool for the characterization of gastroparesis, especially in cases of refractory gastroparesis, to initial traditional management. Most studies have shown an inverse correlation between pyloric distensibility, gastric emptying, and gastroparesis symptoms. This could allow for the individualization of each case by identifying the characteristics of the pylorus, guiding towards a better management strategy, and, in many cases, predicting clinical response.











 text in
text in 



