Introduction
The SARS-CoV-2 infection has introduced new challenges for medical personnel, prompting the global community to unify efforts to mitigate the damage caused by this virus and to establish definitive guidelines for managing a relatively new disease (COVID-19), which primarily affects the respiratory system and occasionally exhibits gastrointestinal involvement1.
While most causes of pneumoperitoneum are of surgical origin, there exists a subset known as spontaneous pneumoperitoneum, where it has been shown that conservative management can avoid the additional morbidity associated with surgical procedures, anesthetic risks, and increased hospital costs2.
Here, we present two cases of patients who were admitted to the emergency department of Hospital Militar Central in Bogotá: a woman and a man with confirmed SARS-CoV-2 infection.
Case presentation
Case 1
An 84-year-old patient with a history of Alzheimer’s disease, oxygen-dependent chronic obstructive pulmonary disease (COPD), degenerative osteoarthritis, cholecystectomy four years ago, and right hip osteosynthesis 45 days prior, presented to the emergency department with a 2-day history of drowsiness associated with irritative urinary symptoms, hematuria, and foul-smelling urine. The patient reported occasional cough, denied abdominal pain, and had regular bowel movements. Upon admission, laboratory tests, a chest X-ray, and computed tomography urography (uro-CT) were requested, and the patient was placed in the observation room.
The complete blood count showed no leukocytosis or neutrophilia, mild anemia, normal renal function and arterial blood gases, hyponatremia of 128 mmol/L, and mildly elevated C-reactive protein at 5.4 mg/dL. The urinalysis suggested infection, with subsequent growth of Enterococcus faecalis in the urine culture. The chest X-ray (Figure 1) revealed diffuse thickening of the central and peripheral interstitium, predominantly on the right side, with patchy areas of ground-glass opacity superimposed on an interstitial pattern, as well as pneumoperitoneum. The uro-CT (Figure 2) showed thickening of the bladder walls without other findings in the urinary tract, and evidence of pneumoperitoneum without identifiable lesions, free fluid, or inflammatory processes.
Due to the imaging findings, the patient was transferred to the COVID isolation area where a polymerase chain reaction (PCR) test was performed, resulting positive for SARS-CoV-2 infection. Consequently, a consultation with the general surgery service was requested due to the presence of pneumoperitoneum.
The patient, bedridden, was assessed using the Barthel index, which scored 40/100. She experienced coughing fits, was asymptomatic gastrointestinally, had a distended and soft abdomen without pain, and showed no signs of peritoneal irritation. However, due to the pneumoperitoneum found in the uro-CT, an abdominal CT with double contrast was requested (Figure 3). This scan revealed bladder wall thickening related to her urinary tract infection, significant pneumoperitoneum, no inflammatory processes or free fluid, and air bubbles in the intestinal walls compatible with pneumatosis cystoides intestinalis. These findings were not present in a contrast-enhanced abdominal CT performed seven months earlier during an acute diarrheal disease study.
The patient was managed expectantly, tolerated oral intake, had regular bowel movements, and never reported abdominal pain. She was administered ampicillin-sulbactam antibiotic therapy for seven days to treat her urinary tract infection, which resulted in an adequate clinical response and improvement in delirium. Follow-up tests showed no leukocytosis or neutrophilia, but correction of her hyponatremia, decreasing C-reactive protein levels, and renal function and electrolytes within normal limits. After 13 days, the patient was discharged with instructions for outpatient follow-up, warning signs, and home oxygen therapy.
Case 2
A 25-year-old patient with a history of inguinal herniorrhaphy, appendectomy, and left lower limb amputation due to a fragmentation weapon injury, presented to the emergency department with a six-day history of dry cough, clear rhinorrhea, fever, and liquid stools. This was followed by left flank pain radiating to the ipsilateral lumbar region. The patient was admitted to the COVID observation area, and laboratory tests and a uro-CT were ordered due to suspicion of urolithiasis.
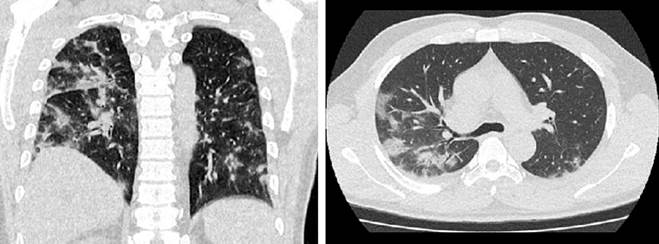
The complete blood count showed leukopenia of 3340 cells/mm³ without neutrophilia, positive C-reactive protein, arterial blood gases with acid-base balance, and slightly elevated lactate at 1.9. The PCR test was positive for SARS-CoV-2 infection. The chest CT scan (Figure 4) showed typical findings of COVID-19 pneumonia, and the uro-CT scan (Figure 5) revealed punctate nephrolithiasis on the right side without obstructive effect and pneumoperitoneum adjacent to the splenic flexure of the colon, prompting a consultation with the general surgery service.
In the clinical evaluation, the patient was clinically stable, with occasional cough, using supplemental oxygen, without tachycardia, and had a soft abdomen with no peritoneal irritation. The double-contrast abdominal CT scan (Figure 6) showed pneumoperitoneum bubbles in the left hypochondrium without inflammatory changes, leading to expectant management. The patient tolerated oral intake well and showed improvement in pain and resolution of liquid stools. Seventy-two hours after admission, the patient experienced resolution of respiratory and gastrointestinal symptoms and was discharged with warning signs and outpatient follow-up.
Discussion
SARS-CoV-2 infection was first identified in December 2019 in Wuhan, Hubei province, China. Initially referred to as the novel coronavirus, the International Committee on Taxonomy of Viruses officially named it SARS-CoV-2, while the World Health Organization (WHO) designated the disease caused by the virus as COVID-191.
It is believed that the virus originated in bats and subsequently transmitted to humans through other animal hosts, exhibiting a high rate of transmissibility. The outbreak was declared a pandemic in March 20201.
SARS-CoV-2 primarily spreads through the respiratory tract via droplets, respiratory secretions, and direct contact. However, the virus has also been isolated from fecal and blood samples, suggesting multiple routes of transmission1. The primary symptoms are respiratory, ranging from mild flu-like symptoms to acute respiratory distress syndrome (ARDS), respiratory failure, multiorgan failure, and even death1.
Common clinical manifestations of COVID-19 include fever (88.7%), cough (67.8%), fatigue (38.1%), sputum production (33.4%), dyspnea (18.6%), sore throat (13.9%), and headache (13.6%). Gastrointestinal symptoms are less common, such as diarrhea (3.8%) and vomiting (5.0%)1. The occurrence of pneumoperitoneum is even rarer, with only one case reported in the literature by Gemio Del Rey and colleagues2, associated with SARS-CoV-2 infection. This rarity highlights the importance of presenting these two new cases of spontaneous pneumoperitoneum associated with COVID-19.
Pneumoperitoneum is defined as the accumulation of free air in the peritoneal cavity3. It was first described by Popper in 19154, and it typically indicates visceral perforation in 85% to 95% of all cases, necessitating surgical intervention3-5. However, in 5% to 15% of cases, pneumoperitoneum does not indicate perforation and arises from other sources that do not require emergency surgery3-5. This subset warrants further exploration.
According to Mularski and colleagues, non-surgical pneumoperitoneum can be classified into five categories based on its origin: thoracic (43.4%), abdominal (30.6%), gynecological (7.6%), and idiopathic (18.4%) (Table 1)5.
Table 1 Causes of Non-Surgical Pneumoperitoneum
| Thoracic | Abdominal | Gynecological | Idiopathic |
|---|---|---|---|
|
Mechanical ventilation: - airway pressure >40-60 cmH2O - high volumes - pre-existing lung disease |
Previous abdominal surgery | Sexual intercourse/oral sex | Cocaine consumption |
| Cardiopulmonary resuscitation | Peritoneal dialysis | Vaginal douching | Scleroderma without pneumatosis cystoides intestinalis |
| Pneumothorax/pneumomediastinum | Gastrointestinal endoscopic procedures | Anatomical anomalies | Decompression diving |
| Airway/chest procedures | Pneumatosis cystoides intestinalis | Pelvic inflammatory disease | Tooth extraction |
| Hysterosalpingography | Other unclear | ||
| Childbirth | |||
| Gynecological medical examination |
Adapted from: Williams NM, et al. Postgrad Med J. 1997;73(863):531-74; Mularski RA, et al. Crit Care Med. 2000;28(7):2638-445.
Thoracic-origin pneumoperitoneum has two proposed mechanisms for the passage of air from the thoracic cavity to the abdominal cavity: direct passage through pleural and diaphragmatic defects, and the classic route through the mediastinum along perivascular connective tissue or larger diaphragmatic portals to the retroperitoneum and finally to the peritoneum; this passage is known as the Macklin effect4. Idiopathic pneumoperitoneum is defined as pneumoperitoneum without a clearly established etiology4,5. Finally, non-surgical abdominal-origin pneumoperitoneum arises from residual air from surgical procedures (60%), peritoneal dialysis (11%-34%), gastrointestinal endoscopic procedures (25%), and is associated with findings of pneumatosis cystoides intestinalis (PCI)4,5. The absorption rate varies for each patient, and pneumoperitoneum can be found in 50% of patients in control tomography on the sixth day5.
As demonstrated in the two presented cases, most patients with spontaneous pneumoperitoneum are asymptomatic or present with vague symptoms, a characteristic that is very clear in patients who do not require surgical management. It is also important to consider the presence of systemic inflammatory response signs in the context of each patient, as the finding of pneumoperitoneum, as noted, is incidental in patients with symptoms secondary to extraintestinal pathologies5.
One of these patients presents findings related to pneumatosis cystoides intestinalis, which recently developed alongside the noted symptoms, as it was not documented in previous CT studies during earlier hospitalizations. Pneumatosis cystoides intestinalis is a rare condition first described by Du Vernoy in autopsies in 1730 and later named by Mayers in 18258. It is defined as the presence of air dissecting the intestinal wall9 and can be divided into two types: primary (15%) and secondary (85%)10. Its severity can range from a benign condition to one that is life-threatening9.
The exact incidence is unknown since most patients are asymptomatic; however, autopsy series reports have found an incidence of 0.03% in the general population9.
There are three main hypotheses regarding the pathophysiology of the condition:
The mechanical theory involves an increase in intraluminal pressure caused by conditions such as intestinal obstructions, inflammatory bowel diseases, mesenteric ischemia, gastrointestinal tract tumors, anorectal surgeries, or endoscopic procedures. This pressure causes mechanical damage to the mucosa, allowing the migration of intraluminal gas into the intestinal wall. However, this theory does not explain the persistence of these cysts once formed.
The pulmonary theory suggests that chronic lung diseases such as COPD, asthma, or pneumonia cause alveolar rupture, leading to mediastinal emphysema and the release of gas along the aorta and mesenteric vessels to the intestinal wall. This theory does not account for the fact that 50% of the gas found in the cysts is hydrogen, a gas not produced by mammals.
The bacterial theory proposes that bacterial translocation to the intestinal wall and subsequent gas production by fermentation can generate this condition; however, the presence of gas-producing bacteria in these cysts has not been confirmed8,10,11.
Additional theories have been described, such as the chemical or nutritional deficit theory, which explains how malnutrition can prevent the digestion of carbohydrates, leading to increased fermentation, large volumes of gas, intestinal distension, ischemia, and subsequently gas dissecting the submucosa. Finally, there have been reports of intestinal pneumatosis secondary to chemotherapy, hormone therapy, and connective tissue diseases, although the pathophysiology remains unknown8.
In a systematic analysis conducted by Wu and colleagues, the average age of patients was found to be 45.3 ± 15.6 years, with variable symptoms. The most common symptoms, in order of frequency, were abdominal pain, diarrhea, distension, nausea and vomiting, bloody diarrhea, mucus in stools, and constipation8. Lesions were primarily located in the colon (46%), followed by the small intestine (27%), a combination of the colon and small intestine (7%), and the stomach (5%)10.
Pneumoperitoneum is a manifestation of an underlying disorder, and its management focuses on treating the underlying cause. Wu and colleagues found that most patients (93%) with primary spontaneous pneumoperitoneum were cured with conservative management, and the effectiveness of antibiotic treatment was only 26.3%8, indicating that the cause is not always infectious.
During the COVID-19 pandemic, cases associating SARS-CoV-2 with imaging findings of intestinal pneumatosis have been reported, similar to other viruses such as the human immunodeficiency virus (HIV), norovirus, and cytomegalovirus12. It has been described that the virus uses the angiotensin-converting enzyme 2 (ACE2) receptor to enter the cell and the transmembrane serine protease 2 (TMPRSS2) to initiate its protein production. These two molecules play a crucial role as they are expressed in both type 2 alveolar cells and enterocytes, suggesting how the virus can invade the gastrointestinal tract mucosa12. Additionally, the virus has been reported to cause degenerative changes in intestinal lymphoid tissue, resulting in a marked decrease in lymphocytes13.
In the two presented cases, it is considered that these patients may have pneumoperitoneum associated with COVID-19. However, it is crucial to differentiate whether the pneumoperitoneum is amenable to medical management. In a case series, Bhayana and colleagues described abdominal imaging findings in COVID-19 and found that 20% (4/20) of their ICU patients had intestinal pneumatosis. Of these, three showed clear signs of ischemia or intestinal necrosis during laparotomy, and only one had pneumatosis cystoides intestinalis14.
While the finding of pneumoperitoneum can be alarming for the medical team, the interpretation and individualization of each case, along with proper evaluation by the surgical team, can help identify patients who are suitable for medical management. This challenges the traditional surgical dogma that “all pneumoperitoneum is surgical”.
Conclusions
SARS-CoV-2 infection, or COVID-19, is a recent disease with high transmissibility that has affected a significant portion of the global population. Healthcare personnel are still learning about it. While respiratory symptoms are well-known, gastrointestinal symptoms such as distension, abdominal pain, diarrhea, and vomiting1 have also been documented. Some patients may present with imaging findings of pneumatosis cystoides intestinalis12,15 and spontaneous pneumoperitoneum2, often asymptomatic. Therefore, it is crucial to discern which patients benefit from surgical management and which from conservative management3-5.
Not all patients with pneumoperitoneum require surgical intervention. Supported by the literature, this article highlights that 5% to 15% of patients benefit from conservative management. It is essential to individualize patient care, conduct a thorough medical history to identify potential causes, and follow up to avoid increasing patient morbidity with unnecessary surgical procedures and costs3-5.
To date, these two reported cases of spontaneous pneumoperitoneum associated with COVID-19 are the first to be published in the Americas. In Europe, only one similar case has been reported by Gemio Del Rey and colleagues2, involving a patient with SARS-CoV-2 infection who required mechanical ventilation, potentially explaining the pneumoperitoneum. However, the two presented cases have a more challenging pathophysiological explanation, hypothesizing the invasion of the virus into gastrointestinal cells via ACE2 and TMPRSS2 receptors found on the surface of enterocytes12, leading to the presentation of pneumatosis cystoides intestinalis and, consequently, pneumoperitoneum. While an intrathoracic cause might be considered due to the pronounced pulmonary presentation in COVID-19 patients, this theory loses weight as the Macklin effect cannot be demonstrated with imaging6.
It is interesting to observe the complex and diverse clinical manifestations of the new SARS-CoV-2 infection, which continues to challenge healthcare personnel. Since there are no definitive management guidelines for the new infection yet, more cases of spontaneous pneumoperitoneum are expected to arise. It is crucial to consider that not all patients presenting with this finding require surgical management. Cases should be individualized, and with appropriate clinical evaluation, the risk of morbidity and mortality from unnecessary surgical interventions can be reduced.











 text in
text in