Introduction
Adenocarcinoma is the most common solid tumor lesion in the pancreas, accounting for approximately 85% of cases, followed by neuroendocrine tumors, which make up 5%, and only 2% of cases are metastatic lesions. The remaining cases include other types of lesions such as pancreatic lymphoma, solid pseudopapillary tumor, pancreatoblastoma, and neurogenic tumors1-5. The primary tumors most likely to metastasize to the pancreas include renal cell carcinoma, lung cancer, melanoma, breast cancer, colon cancer, bladder cancer, and others1,2,5-7. Biliopancreatic endoscopic ultrasound is considered the most effective study for detecting pancreatic masses compared to other imaging studies, with sensitivity and specificity rates of 84.9% and 95.9%, respectively, for detecting pancreatic metastases8,9.
Moreover, fine-needle aspiration guided by endoscopic ultrasound is the first-line diagnostic tool for the histopathological identification of these lesions, with reported accuracy rates of 93.4% for lesions larger than 20 mm, 83.5% for lesions between 10 mm and 20 mm, and 82.5% for lesions smaller than 10 mm10. Complications from the procedure are infrequent, with an approximate rate of 2.5%, the most common being abdominal pain, and rare cases including pancreatitis, bleeding, perforation, and biliary peritonitis11-13. Additionally, this method has been shown to have advantages over other sample acquisition techniques such as percutaneous puncture and endoscopic retrograde cholangiopancreatography, whether used as a first diagnostic option or as an alternative after the failure of these procedures. Studies have found false negatives that were later confirmed as positive by fine-needle aspiration(12,14). This article describes the case of a patient with small cell lung carcinoma metastasis in the uncinate process and body of the pancreas.
Clinical case
A 58-year-old male patient initially presented in August 2021 with an episode of hemoptysis, constitutional symptoms, progressive dyspnea, and decreased functional status. A contrast-enhanced computed tomography (CT) scan of the chest and abdomen revealed a large mediastinal mass infiltrating and compressing the esophagus, vascular, and bronchial structures, with no evident abdominal infiltration (Figures 1 and 2). A biopsy confirmed a small cell lung neuroendocrine carcinoma through immunohistochemistry. Staging studies at that time were negative for metastatic lesions, making the patient a candidate for chemotherapy and radiotherapy. He completed three cycles of chemotherapy with cisplatin and etoposide, along with 23 sessions of consolidative radiotherapy to the mediastinum, between December 2021 and March 2022, achieving an adequate response at that time.
Author’s File.
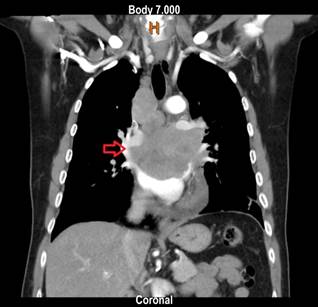
Figure 1 Contrast-enhanced chest CT, coronal view. Mediastinal mass compressing and infiltrating the esophagus and vascular structures.
Author’s File.
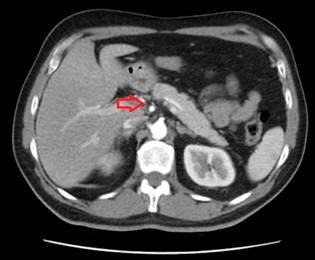
Figure 2 Contrast-enhanced abdominal CT, transverse view. Pancreas without evidence of tumor lesions or infiltration.
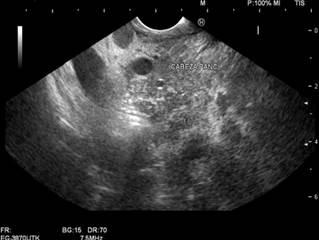
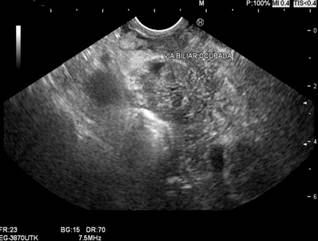
The patient subsequently returned to the emergency department in May 2023 due to progressive dyspnea. A chest CT scan revealed mediastinal involvement from a mass infiltrating and compressing the vascular structures, esophagus, and bronchi. An abdominal CT scan showed a tumor lesion in the uncinate process extending to the body of the pancreas (Figures 3 and 4). Consequently, a linear biliopancreatic endoscopic ultrasound was performed, identifying a tumor lesion measuring 62 x 54 mm in diameter, originating from the uncinate process and extending to the body of the pancreas (Figure 5). The ultrasound also revealed periportal lesions compatible with multiple adenopathies and distal bile duct invasion without secondary dilation (Figure 6).
Author’s File.
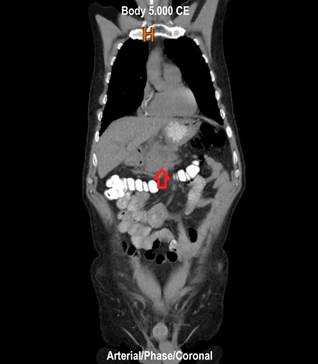
Figure 3 Contrast-enhanced abdominal CT, coronal view. Mass occupying the uncinate process and extending to the body of the pancreas.
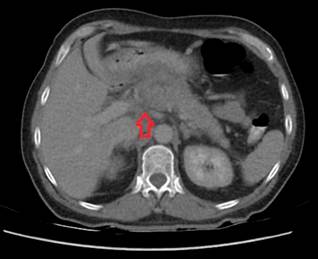
Author’s File.
Figure 4 Contrast-enhanced abdominal CT, transverse view. A window from the stomach for eventual ultrasound-guided biopsy is observed.
Author’s File.
Figure 5 Linear biliopancreatic endoscopic ultrasound. Mass originating from the uncinate process and extending to the body of the pancreas.
Author’s File.
Figure 6 Linear biliopancreatic endoscopic ultrasound. Partial invasion of the bile duct.
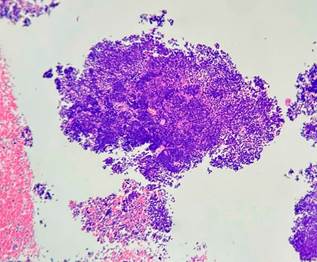
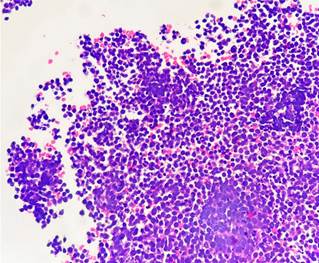
A biopsy was performed using endoscopic ultrasound guidance, employing a transgastric approach with a modified wet suction technique and a 19G needle. The sample obtained was analyzed for cytopathology and histopathology, leading to a final diagnosis of a high-grade malignant tumor with small round cell morphology (Figures 7 and 8). The patient received an additional dose of chemotherapy in the emergency department, which resulted in an unfavorable clinical outcome and a decline to NYHA functional class III/IV. As a result, it was ultimately determined that the patient was a candidate for a home palliative care package.
Author’s File.
Figure 7 Hematoxylin and eosin stain, 40x. Small cells with hyperchromatic round nuclei arranged in sheets, with frequent mitotic figures and cellular debris.
Discussion
Biliopancreatic endoscopic ultrasound is regarded as the most effective imaging technique for detecting biliopancreatic lesions15. Its sensitivity for detecting pancreatic masses is significantly higher compared to contrast-enhanced CT, with rates of approximately 98% versus 74%, respectively, particularly for lesions smaller than 2 cm10,11. The sensitivity and specificity for detecting pancreatic metastases using endoscopic ultrasound are 84.9% and 95.9%, respectively16. Moreover, fine-needle aspiration biopsy guided by endoscopic ultrasound is considered the first-line histopathological diagnostic method for pancreatic lesions, boasting high sensitivity and specificity for detecting metastases, ranging from 84% to 92% and 96% to 98%, respectively, with an overall diagnostic accuracy of 89.3% to 93.3%1,4,7-9,16-19. Reported accuracy is 93.4% for lesions larger than 20 mm, 83.5% for lesions between 10 mm and 20 mm, and 82.5% for lesions smaller than 10 mm10. While CT and MRI are recommended for diagnosis and staging, they perform poorly in detecting small masses compared to endoscopic ultrasound10,18,20-24. Sensitivity is influenced not only by lesion size but also by factors such as solid consistency and location in the neck and body of the pancreas, which enhance diagnostic potential9,25. Some literature suggests endoscopic ultrasound as a screening tool for high-risk patients, such as those with a first-degree family history of pancreatic cancer or genetic mutations and syndromes associated with lesion development, like Peutz-Jeghers syndrome26,27.
Pancreatic metastases are rare, with limited reports in the literature, and their prevalence is approximately 2%1-5. The primary tumors most frequently metastasizing to the pancreas include cancers of the kidney, lung, gastrointestinal tract, and breast1,2,5-7. Most metastatic lesions appear as solid, hypoechoic, and hypovascularized1,5. A recent retrospective study involving 2560 patients found that 28 had pancreatic metastases (1%), with only 20 undergoing endoscopic ultrasound. Among these, five had lung cancer (2 with small cell carcinoma and 3 with squamous cell carcinoma). The small cell carcinoma lesions showed hypervascularization, contrasting with the predominantly hypoechoic, hypovascularized lesions1. In this case, conventional linear biliopancreatic endoscopic ultrasound was performed along with fine-needle aspiration, revealing solid, hypoechoic lesions without Doppler signal, aligning with most studies and differing from the last mentioned study.
It can be asserted that the presented case corresponds to metastasis of small cell lung neuroendocrine carcinoma to the pancreas. At the time of diagnosis, imaging showed only a mediastinal mass with local involvement of the esophagus and vascular structures, without any intra-abdominal lesions suggestive of neoplasia or other findings in other systems that could indicate a primary tumor different from the lung. Moreover, biopsy results along with immunohistochemistry confirmed the pulmonary origin. Additionally, the sample taken from the pancreatic lesion via endoscopic ultrasound showed histopathological results consistent with a small round cell malignant tumor, correlating with the pathology findings of the lung mass. This rules out the possibility of a new primary pancreatic tumor. This latter biopsy is crucial for determining the prognosis and guiding treatment or interventions that would benefit the patient.
Conclusions
Biliopancreatic endoscopic ultrasound is an essential tool for diagnosing pancreatic tumor lesions. It has demonstrated high diagnostic performance and safety when obtaining biopsy samples. It should be considered a first-line diagnostic tool for such lesions. Pancreatic metastases are very rare, and obtaining a histopathological sample is crucial for the definitive diagnosis of these lesions. Biliopancreatic endoscopic ultrasound enables this by allowing guided puncture, providing greater accuracy and fewer complications compared to other diagnostic methods. The imaging characteristics of the metastatic small cell carcinoma lesion in the pancreas in this clinical case, obtained via ultrasound, align with the majority of studies with similar histopathological results.











 text in
text in