Introduction
According to the World Health Organization (WHO), obesity is a chronic disease defined by a body mass index (BMI) ≥30 kg/m²1. It is classified into class I obesity (BMI: 30-34.9 kg/m²), class II (BMI: 35-39.9 kg/m²), and class III (BMI: ≥40 kg/m²). Its prevalence has increased over the last 50 years, and it is currently estimated that over 650 million adults suffer from obesity. As such, it is considered a global public health issue, associated with multiple comorbidities. In addition to the underlying social burden, it generates high costs for individuals, their surroundings, and healthcare systems2-5. Triggers for obesity, besides dietary habits, include social, environmental, and physical factors that individuals experience6.
In Colombia, the National Survey of Nutritional Situation (ENSIN in Spanish) estimated that obesity in adults rose from 13.7% in 2005 to 18.7% in 20157. According to data from the World Obesity Federation, the overall prevalence in 2018 was 21.3%, with a higher rate in women than in men (22.5% vs. 14.4%)8. Obesity is associated with the occurrence of cardiovascular diseases as well as others, such as osteoarthritis, fatty liver, depression, cancer, and gastrointestinal conditions, including esophageal, gastric, small intestine, colon, and pancreatic diseases9.
Lifestyle modification interventions have been the cornerstone of obesity management, showing greater short-term effectiveness with an average weight loss of 3% to 5%. However, in the long term, patients regain up to 80% of the weight lost10-12. Consequently, maintaining weight loss is a challenge for both patients and physicians. Clinical practice guidelines recommend a multimodal treatment approach, which includes the use of medications and the performance of medical-surgical procedures, such as bariatric surgery and bariatric endoscopy (BE), to help patients maintain weight loss and achieve treatment goals11.
Currently, BE procedures, being minimally invasive, are considered to bridge the gap between medical and surgical interventions for obesity treatment. For this reason, gastroenterologists have become part of multidisciplinary teams offering less invasive management alternatives with success rates comparable to conventional treatments. These options are especially suitable for patients who are not candidates for surgical procedures or have a high surgical risk13, for those who have undergone surgery but developed complications (5%-25%), and for managing patients who have failed to maintain optimal weight in the long term14.
Medications for the Management of Obesity
There are several medications available for the treatment of obesity. Among the most commonly used therapeutic options are glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide and semaglutide, as well as other drugs like phentermine-topiramate, naltrexone-bupropion, and orlistat. These medications are indicated for patients with class I obesity or for those with a BMI ≥27 kg/m² who have an obesity-related health condition. In Colombia, two medications are approved for the management of obesity: liraglutide and orlistat.
The effectiveness of different therapeutic options in achieving a weight loss of ≥5% was evaluated in a recent meta-analysis. It found that, in order of efficacy, phentermine-topiramate showed the greatest weight loss (odds ratio [OR]: 8.02), followed by GLP-1 receptor agonists (OR: 6.33), and naltrexone-bupropion (OR: 2.69). Additionally, there was a higher risk of adverse events with GLP-1 agonists and orlistat15. It is important to note that the effectiveness of anti-obesity medications in reducing weight has improved with the development of new therapies. First-generation drugs achieved weight loss of 5% to 10% of initial body weight, while newer treatments can result in reductions of up to 10%-15%16.
In addition to weight loss, GLP-1 medications have been reported to offer protective effects against cardiovascular events and mortality in patients with type 2 diabetes mellitus17. The SELECT study demonstrated benefits in reducing cardiovascular mortality, myocardial infarction, and stroke with weekly doses of 2.4 mg semaglutide compared to placebo in overweight or obese patients without diabetes but with underlying cardiovascular disease16,18-20. New pharmacological therapies are under study, including tirzepatide, retatrutide, and orforglipron, which have shown weight reductions of 20.9%, 24.2%, and 14.7%, respectively, compared to placebo12,21,22.
Table 1 presents the characteristics of the medications commonly used in the management of obesity.
Table 1 Medications Frequently Used for the Management of Obesity
| Medication/Mechanism of Action | Average Weight Loss Compared to Placebo During the First Year | Contraindications | Adverse Effects | Mode of Administration |
|---|---|---|---|---|
|
Liraglutide (GLP-1 analogue) - Central appetite control, slows gastrointestinal transit, and reduces glucose levels. |
- 6% greater than placebo and 4% greater than placebo in patients with T2DM. - Has shown reduction in mortality and cardiovascular disease events in patients with T2DM. |
Pregnancy, breastfeeding, family history of medullary carcinoma or multiple endocrine neoplasia syndrome. | Nausea, vomiting, diarrhea, dyspepsia, abdominal pain, constipation, and headache. |
- Subcutaneous injection. - Daily administration with weekly dose escalation (0.6 mg; 1.2 mg, 1.8 mg, 2.4 mg, 3 mg). |
|
Semaglutide* (GLP-1 receptor analogue) - Central appetite control, slows gastrointestinal transit, and reduces glucose levels. |
- 14.9% on average compared to 2.4% with placebo in non-diabetic patients with overweight or obesity. - Has shown reduction in mortality and cardiovascular disease events in both T2DM and non-diabetic patients with established cardiovascular disease and overweight or obesity. |
Pregnancy, breastfeeding, family history of medullary carcinoma or multiple endocrine neoplasia syndrome. | Pancreatitis, nausea, vomiting, diarrhea, dyspepsia, abdominal pain, constipation, and headache. |
- Subcutaneous injection. - Weekly administration with weekly dose escalation (0.25 mg, 0.5 mg, 1.0 mg, 1.7 mg, 2.4 mg). |
|
Phentermine and Topiramate - Central appetite control. - Sympathomimetic and GABA receptor activation (phentermine). - Inhibition of carbonic anhydrase receptor (topiramate). |
- 9% greater than placebo and 6.7% greater than placebo in patients with T2DM. | Pregnancy, breastfeeding, history of glaucoma, kidney stones, cardiovascular disease, uncontrolled hypertension. | Paresthesias, altered taste (dysgeusia), memory loss, depression, and fetal malformations. |
- Oral daily intake. - Starts with low doses (3.75 mg/23 mg) with gradual increases after day 14. |
|
Naltrexone and Bupropion - Central appetite control. - Opioid receptor antagonist (naltrexone). - Dopamine and norepinephrine reuptake inhibitor (bupropion). |
- 5% greater than placebo and 3.2% greater than placebo in patients with T2DM. | Pregnancy, breastfeeding, uncontrolled hypertension, seizures, bipolar disorder, bulimia, anorexia, opioid use. | Dry mouth, nausea, vomiting, irritability, headache, and insomnia. |
- Oral daily intake. - Starts with 8 mg/90 mg during the first week with weekly dose escalation until reaching 2 tablets in the morning and 2 in the evening by the fourth week of treatment. |
|
Orlistat - Central appetite control and stimulation of norepinephrine, serotonin, and dopamine release. - Inactivation of gastric and pancreatic lipase. |
- 2.9% weight reduction greater than placebo. | Pregnancy, breastfeeding, and patients with cholestasis. | Steatorrhea, fecal urgency, and fat-soluble vitamin deficiencies. |
- Oral daily intake. - 60-120 mg three times a day. |
*As of now, semaglutide is approved in Colombia for the management of diabetes, but not as a weight control medication. T2DM: type 2 diabetes mellitus; GLP-1: glucagon-like peptide 1. Adapted and modified from: Schmitz SH, and colleagues. Gastrointest Endosc Clin N Am. 2023;52:661-8041.
Bariatric Endoscopy for the Management of Obesity
Bariatric endoscopy (BE) procedures have been developed to enhance treatment options for individuals who are not candidates for conventional bariatric surgery. These procedures are less invasive, shorter in duration, performed on an outpatient basis, and offer quicker recovery times17,23. Among these interventions are the use of the intragastric balloon (IGB), endoscopic sleeve gastroplasty (ESG), primary obesity surgery endolumenal (POSE), and post-bariatric revision or repair procedures such as transoral outlet reduction (TORe).
Intragastric Balloon
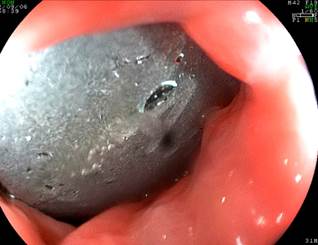
The use of IGB has demonstrated a reduction in the amount of food consumed, secondary to reduced gastric capacity and delayed gastric emptying. Currently, a saline-filled balloon containing methylene blue is used, and it is removed after 6-12 months. Air-filled balloons have shown less weight loss (3-4 kg on average) compared to liquid-filled balloons24,25. The IGB placement is performed in an endoscopy unit, is an outpatient procedure, and lasts approximately 30 minutes, with discharge occurring one to two hours afterward. Figures 1, 2,3, and 4 illustrate key moments and steps to ensure the proper placement of an IGB.
Author’s file.
Figure 1 Subcardial positioning as a reference point for initiating balloon inflation.

Author’s file.
Figure 2 Retroflexion view as an optional maneuver for full visualization of the balloon during inflation with saline solution and methylene blue.
According to the American Gastroenterological Association (AGA), IGB placement is recommended for overweight and obese individuals who have failed conventional treatments. The Brazilian Consensus on Endoscopic Gastroplasty (CBGE) recommends IGB placement in patients with overweight and class 1, 2, and 3 obesity, with no upper age limit for the procedure26. The prophylactic administration of proton pump inhibitors (PPIs) and antiemetics should be considered during the perioperative period and up to two weeks after device placement, due to the potential for abdominal pain, vomiting, and gastroesophageal reflux disease (GERD)27.
Approximately 7% of patients who have undergone IGB placement experience dehydration, 2% are readmitted to medical services, and 1% require reintervention24,28. Complications have been reported in fewer than 2% of patients, including hyperinflation, spontaneous rupture, migration, gastric ulcer, bleeding, pancreatitis, and premature or early removal25.
From a technical standpoint, there are different types of devices available. Table 2 describes four therapeutic alternatives commonly used in Colombia.
Table 2 Characteristics of Intragastric Balloons Commonly Used in Colombia
| Balloon Type | Material | Technical Aspects for Placement and Removal | Reported Weight Loss | Reported Complications29 |
|---|---|---|---|---|
| Orbera | Silicone |
- Placement via endoscopy - Filled with 400-700 mL of saline solution - Removal via endoscopy after 6-12 months |
- 15.3% body weight loss (Orbera six months) compared to 14.7% (Orbera 365) - 26.5% excess weight loss |
- 1.4%: device migration - 2%: gastric ulcer - 18.3%: reflux - 1.1%: intolerance |
| Silimed | Silicone |
- Placement via endoscopy - Filled with 650 mL of saline solution - Removal via endoscopy after six months |
- Initial weight: 107.7 ± 25.1 kg, weight after six months of treatment: 89.4 kg - Initial BMI: 35.7 ± 5.7 kg/m², BMI after six months of treatment: 31.8 kg/m² |
- 21%: epigastric pain - 3.8%: device deflation |
| Spatz 3 | Silicone |
- Placement via endoscopy - Filled with 300-900 mL of saline solution - Adjustable valve to optimize results - Removal via endoscopy after 12 months |
- 20.1% body weight loss - 45.8% excess weight loss |
- Gastric ulcer, migration, intolerance in 4.7% of cases |
| Elipse Allurion | Polyurethane |
- Does not require endoscopy, administered orally - Filled with 550 mL of water - Naturally expelled after four months |
- 11.6% body weight loss - 26% excess weight loss |
- 2.9%-7.03%: device intolerance - 0.6%: device deflation |
Adapted from: Ameen S, and colleagues. Expert Rev Med Devices. 2024;21(1-2):37-5429.
Endoscopic Sleeve Gastroplasty (ESG)
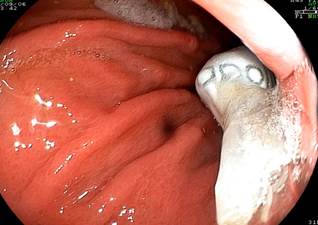
ESG is the most commonly performed gastric remodeling procedure and involves placing several continuous sutures along the greater curvature of the stomach; a second layer of linear sutures can also be placed as reinforcement (Figure 5). Current evidence shows total weight loss effectiveness between 16% and 20% at 12 months30-32, along with statistically significant reductions in blood pressure, waist circumference, triglycerides, glycated hemoglobin (HbA1c) levels, and hepatic steatosis index23. Although this weight loss is less than that achieved with conventional surgery, ESG has a lower probability of adverse events and shorter recovery times. Additionally, it does not induce de novo gastroesophageal reflux disease (GERD) symptoms nor worsen existing GERD33,34.
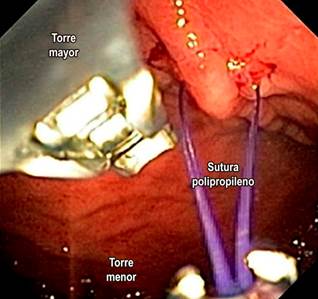
Figure 5 Gastric endosuturing with the Overstitch method. Photo courtesy of Dr. Valeria Atenea Costa.
According to the U.S. Food and Drug Administration (FDA), candidates for ESG are patients with a BMI between 30 and 50 kg/m², while the CBGE considers the procedure for patients with a BMI of 27 kg/m²26. Currently, there is no established maximum age for performing the procedure, so it is recommended that each patient be evaluated individually.
Contraindications include active gastric ulcers, congestive gastropathy, gastric polyposis, giant hiatal hernia, gastric or esophageal varices, and uncontrolled psychiatric disorders.
ESG involves gastric restriction through full-thickness or transmural sutures placed endoscopically23,35 and is performed under general anesthesia25,36 (Figure 6). Initially, an endoscopic evaluation is required to rule out contraindications and determine potential suture sites in the gastric chamber walls. The procedure begins distally and proceeds proximally, maintaining a U suture pattern. The goal is to reduce gastric capacity by 70%-80%, transforming the gastric fundus into a pouch that limits tolerated volume and induces gastric restriction, resulting in delayed or impaired gastric emptying for solids (Figure 7). During and after the procedure, antibiotics, antiemetics, and analgesics are typically administered.
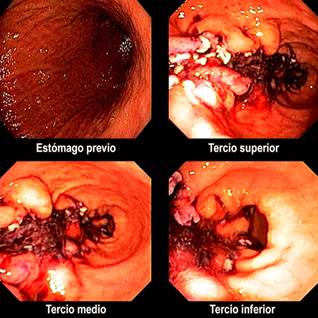
Figure 6 Final endoscopic view of an endoscopic sleeve gastroplasty. Images courtesy of Dr. Andrés Rodríguez.

Figure 7 Gastric restriction with ESG. Adapted from: Barrichello S, and colleagues. Gastrointestinal Endoscopy. 2019;90(5):770-78042.
Other Treatment Alternatives
Some patients who undergo bariatric surgery experience weight regain and require post-bariatric repair or revision. The TORe technique is a minimally invasive alternative in which an endoscopic suture is placed at the gastrojejunal level in patients with Roux-en-Y bypass to reduce the size of the dilated stoma following surgery, with or without suturing the gastric pouch (Figure 8).

Figure 8 Video showing post-bariatric endoscopic repair after gastric bypass: TORe method with argon plasma and gastric endosuturing. Video courtesy of Dr. Jesús Rodríguez and Dr. Valeria Atenea Costa. Available at https://youtu.be/pbs6ezdDQ9M
Another recent alternative for managing obesity is primary surgery with POSE endoplication, which uses an incisionless technique to create folds in the stomach body, leading to reduced capacity and delayed gastric emptying. Preliminary reports have shown a reduction in excess weight at thee and six months by 42% and 48%, respectively37,38.
Finally, the duodenal mucosal resurfacing (DMR) technique uses a catheter for hydrothermal ablation of the duodenal mucosa, aimed at improving glycemic control by restoring cellular anatomy and modulating the endocrine response in patients with diabetes39,40.
These therapeutic alternatives are still under development, and their efficacy and safety profiles in different population groups remain to be fully established.
Conclusions
Given the current scenario, with a growing number of patients who are overweight or obese, gastroenterologists play a crucial role in managing and monitoring patients who have not undergone surgical interventions or who have experienced poor outcomes in sustaining weight loss and/or complications from such procedures. These issues must be managed in collaboration with the surgical team.
It is important to highlight the unmet needs in obesity control for a large number of patients. Pharmacotherapy as monotherapy and lifestyle interventions have shown modest short- to medium-term results, which are unsustainable in the long term. On the other hand, while bariatric surgery is effective, it remains inaccessible to many patients due to its cost, in addition to the potential complications and its invasive nature. This review outlines minimally invasive, reversible, effective, and safe bariatric endoscopy (BE) alternatives. However, additional studies are needed to determine long-term efficacy and outcomes regarding metabolic adjustments and comorbidities in Latin American populations, considering the specific social, cultural, and biological aspects of these communities. Nonetheless, it is clear that the future of obesity medicine lies in a multidisciplinary approach and close follow-up, requiring the combination of various treatment strategies.











 text in
text in